SER-109, an Oral Microbiome Therapy, Decreases Recurrent Clostridiodes difficile Infection
 Jessica R. Allegretti, MD, MPH
Jessica R. Allegretti, MD, MPH
Associate Director, Crohn’s and Colitis Center, Division of Gastroenterology,
Hepatology and Endoscopy, Department of Medicine, Brigham and Women’s
Hospital, Harvard Medical School, Boston, MA
This summary reviews Feuerstadt P, Louie TJ, Lashner B, et al. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N Engl J Med 2022; 386(3): 220-229. PMID: 35045228.
Correspondence to Jessica R. Allegretti, MD, MPH, Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Will spore-forming bacteria, which may compete metabolically with Clostridioides difficile spores for essential nutrients and modulate bile-acid profiles to re-establish colonization resistance to reduce recurrent C. difficile infection (CDI) after successful antibiotic therapy?
Design: Randomized, double-blind, placebo-controlled trial.
Setting: Fifty-six US and Canadian Sites from July 2017 through September 2020.
Patients: The trial included adults with 3 or more confirmed episodes of CDI within 12 months, which was defined as 3 or more unformed bowel movements over 2 consecutive days, positive C. difficile toxin test, and resolution of symptoms while receiving 10-21 days of antibiotic therapy. Patients were required to test positive for C. difficile toxin by enzyme immunoassay. A total of 281 patients were screened and 182 underwent randomization. Mean age was 65.5 years; 59.9% female; 93% White; 73.1% previously treated with vancomycin, and 26.9% previously treated with fidaxomicin before randomization.
Exposure: Patients were randomized 1:1 to receive SER-109, an investigational microbiome therapeutic composed of live purified Firmicutes bacterial spores with approximately 3×107 spore colony-forming units or 4 placebo capsules, daily, for 3 consecutive days. All patients were given 10 ounces of magnesium citrate the night before treatment to minimize persistent active antibiotic in the colon.
Outcome: Sustained clinical response defined as no recurrence of CDI up to 8 weeks after dosing (the primary efficacy end point).
Data Analysis: Efficacy analyses were performed in the intention-to-treat population, which included all patients who underwent randomization. Patients who were lost to follow-up, discontinued participation in the trial prematurely, or died without a recurrence of CDI before 8 weeks after treatment were defined as having a CDI recurrence. Cochran–Mantel–Haenszel test was used to calculate the relative risk of recurrent CDI with SER-109 vs placebo, stratified according to age (<65 years or ≥65 years) and previous antibiotic regimen for the qualifying episode (vancomycin or fidaxomicin).
Results: The percentage of patients with a CDI recurrence was lower in the SER-109 group compared to placebo (12% vs 40%, respectively; RR = 0.32; 95% CI: 0.18-0.58). Most recurrences (75%) occurred within 2 weeks after treatment administration. No serious adverse events were assessed as being related to SER-109. The common adverse events were mild GI disorders. Funding: Seres Therapeutics, manufacturer of Ser-109.
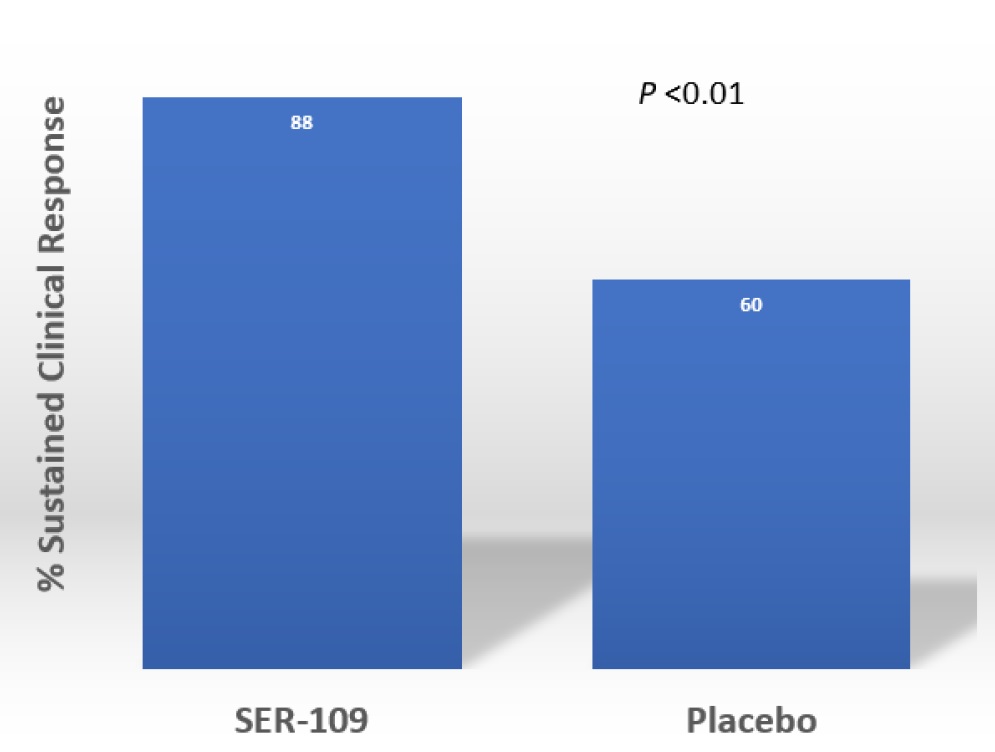
Figure 1. Primary efficacy outcome
COMMENTARY
Why Is This Important?
Recurrent CDI remains a huge public health issue with cases continuing to rise. While fecal microbiota transplantation (FMT) has been shown to be effective for the prevention of recurrent CDI, there are currently no effective FDA-approved therapies1. In addition, restrictions brought on by the pandemic have limited access to FMT2. Therefore, new treatments to minimize CDI are sorely needed.
An FDA application for approval to use SER-109 will likely be submitted in 2022. The current study demonstrates the efficacy of starting treatment for recurrent CDI with antibiotics followed by a microbiome therapeutic to assist with microbiome repair to minimize recurrent infection.
Key Study Findings
Patients treated with SER-109 had much lower risk of recurrent CDI compared to placebo (12% vs 40%, respectively; RR = 0.32; 95% CI: 0.18-0.58) after a course of standard of care antibiotics. There were no serious adverse events found to be related to SER-109 observed through week 8.
Caution
While the study did show superiority of SER-109 over placebo, the trial design may not be representative of clinical practice. Notably, the protocol requires 10 ounces of magnesium citrate on the evening before initiating SER-109 to improve efficacy by ensuring adequate antibiotic wash out. However, it’s unclear how failure to do this bowel prep will impact efficacy. This protocol also mandated enzyme immunoassay toxin positivity as entry criteria and most centers around the country are still using PCR testing.
My Practice
I am currently still off “traditional” FMT under the FDA policy of enforcement discretion for those with 3 or more confirmed episode of CDI. Instead, our regional Center of Excellence for FMT gets properly screened and frozen stool from OpenBiome’s biobank. However, OpenBiome has limited supply and is only providing material to these Centers of Excellence. Therefore, if your patient has recurrent CDI, then I suggest referral to a regional Center of Excellence (and OpenBiome’s website provides a list of these sites) or academic medical centers with FMT programs. You can also check clinicaltrials.gov for active trials of investigational microbiome agents.
SER-109 is currently under review by the FDA for approval. The FDA will probably rescind their current policy allowing FMT when we have FDA-approved microbiome therapeutics, and I certainly intend to use them. The biggest challenge, as with many things in medicine, will be insurance coverage and cost to the patient.
For Future Research
Several microbiome-based therapeutics are currently under investigation. We are likely to have several options for the prevention of recurrent CDI in the near future. Future research on microbiome-based therapeutics, both full spectrum and defined consortia, in other chronic diseases are definitely needed, too. Some studies are already underway with inflammatory bowel disease3.
Conflicts of Interest
Dr. Allegretti reports serving as a consultant for Seres Therapeutics, Finch Therapeutics, and is a scientific advisor for Openbiome.
REFERENCES
- Allegretti JR, Kassam Z, Osman M, et al. The 5D framework: a clinical primer for fecal microbiota transplantation to treat Clostridium difficile infection. Gastrointest Endosc 2018; 87:18-29.
- Ng SC, Chan FKL, Chan PKS. Screening FMT donors during the COVID-19 pandemic: a protocol for stool SARS-CoV-2 viral quantification. Lancet Gastroenterol Hepatol 2020; 5:642-643.
- Henn MR, O’Brien EJ, Diao L, et al. A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology 2021; 160:115-127 e30.

