Subcutaneous Risankizumab Is Effective and Safe for the Maintenance of Moderate-to-Severe Crohn’s Disease
 Rahul S. Dalal, MD, MPH1 and Jessica R. Allegretti, MD, MPH, FACG2
Rahul S. Dalal, MD, MPH1 and Jessica R. Allegretti, MD, MPH, FACG2
1Division of Gastroenterology, Hepatology and Endoscopy, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
2 Medical Director, Crohn’s and Colitis Center, Division of Gastroenterology, Hepatology and Endoscopy, Department of Medicine, Brigham and Women’s Hospital; Assistant Professor of Medicine, Harvard Medical School, Boston, MA
This summary reviews Ferrante M, Panaccione R, Baert F, et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal Phase 3 FORTIFY maintenance trial. Lancet 2022;399(10340):2031-2046.
Access the article through PubMed
Correspondence to Jessica Allegretti, MD, MPH. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Is subcutaneous Risankizumab (Skyrizi; AbbVie Pharmaceuticals, San Francisco, CA), a selective anti-interleukin (IL)-23 antibody, effective and safe for the maintenance of clinical remission of moderate-to-severe Crohn’s disease?
Design: Phase 3 randomized, double-blind, placebo-controlled, 52-week maintenance withdrawal trial (FORTIFY).
Setting: The study was conducted in 273 clinical centers in 44 countries across North America, South America, Africa, Europe, Australia, and the Asia-Pacific regions.
Patients: In total, 542 patients with moderate-to-severe Crohn’s disease and initial clinical response (defined as > 30% reduction in mean stool frequency and mean daily abdominal pain score in 7 days prior to assessment) or clinical remission (see definition in Outcomes) at week 12 or week 24 after intravenous (IV) risankizumab induction therapy from the ADVANCE and MOTIVATE trials were enrolled in FORTIFY between April 2018 and April 2020.
Interventions: All patients were randomized 1:1:1 to 360 mg of subcutaneous risankizumab, 180 mg of subcutaneous risankizumab, or subcutaneous placebo (referred to as “withdrawal”) every 8 weeks.
Outcomes: Co-primary endpoints included week 52 clinical remission (stratified by 2 definitions of clinical remission: 1. Crohn’s disease activity index [CDAI] <150 or 2. mean liquid/soft stool frequency < 2.8/day and abdominal pain scores <1 and not worse than baseline) and endoscopic response (decrease in Simple Endoscopic Score for Crohn’s Disease [SES-CD] by 50% from baseline). Secondary endpoints included stool frequency remission, abdominal pain remission, Crohn’s Disease activity index (CDAI) response, endoscopic remission, and deep remission (clinical and endoscopic remission), among other outcomes. Adverse effects were also assessed through 52 weeks.
Data Analysis: Categorical primary and secondary endpoints were analyzed using the Cochran-Mantel-Haenszel test using a 2-sided significance level of 0.05. Continuous endpoints were assessed using mixed-effect models for repeated measures or analysis of covariance models in the absence of repeated measures.
Funding: AbbVie Pharmaceuticals, manufacturer of risankizumab.
Results: For 360 mg risankizumab versus placebo, risankizumab was associated with higher rates of clinical remission (CDAI clinical remission 52% vs 41%, P <0.05 and stool frequency/abdominal pain score clinical remission 52% vs 40%, P <0.05) and endoscopic response (47% vs 22%, P<0.05). For 180 mg risankizumab versus placebo, risankizumab was associated with higher rates of CDAI clinical remission and endoscopic response but not stool frequency/abdominal pain score clinical remission. Key outcomes from the study are summarized in Figure 1.
Among patients with previous biologic failure, clinical remission and endoscopic response were reduced in all groups (CDAI clinical remission: 48% risankizumab 360 mg, 49% risankizumab 180 mg, 35% placebo; stool frequency/abdominal pain score clinical remission: 48%, 41%, and 34%; endoscopic response: 44%, 41%, and 20%). Adverse events were similar across groups, and most commonly included worsening of Crohn’s disease, arthralgia, and headache.
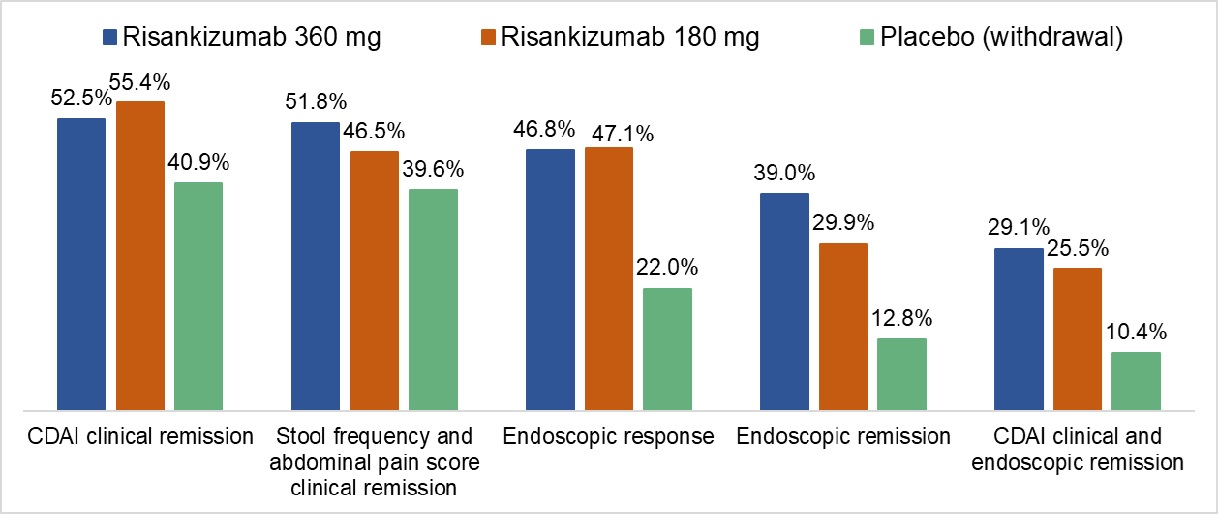 Figure 1. Key Outcomes at 52 Weeks.
Figure 1. Key Outcomes at 52 Weeks.All comparisons are statistically significant with the exception of risankizumab 180 mg versus placebo for stool frequency and abdominal pain score clinical remission only. CDAI, Crohn’s Disease activity index.
COMMENTARY
Why Is This Important?
Current biologic therapies for moderate-to-severe Crohn’s disease target tumor necrosis factor α (infliximab, adalimumab), α4β7 integrin (vedolizumab), and IL-12 and 23 (ustekinumab). Many patients do not have adequate response to existing biologic therapies, therefore additional agents with distinct mechanisms of action are needed. IL-23, a cytokine felt to be associated with chronic bowel inflammation, has been found in high concentrations in the gut mucosa of patients with Crohn’s disease.1,2 Ustekinumab (Stelara; Janssen Pharmaceuticals, Beerse, Belgium), which targets both IL-12 and 23 has been shown to be effective for induction and maintenance of moderate-to-severe Crohn’s disease and ulcerative colitis.3 Selective IL-23 inhibition may be a reasonable target for patients with prior non-response or loss of response to ustekinumab or other biologics.
Risankizumab is a selective anti-IL-23 monoclonal antibody that has recently been shown to be safe and effective for the induction of moderate-to-severe Crohn’s disease in phase 3 trials (ADVANCE and MOTIVATE) compared to placebo.4 (See preceding summary in this issue for full details about these randomized controlled trials (RCTs) and the mechanism of action for Risankizumab.) Additional clinical trial data is necessary to demonstrate the safety and efficacy of risankizumab during maintenance of moderate-to-severe Crohn’s disease.
This RCT included 542 patients with initial response to risankizumab during induction. These patients were then randomized to 360 mg risankizumab, 180 mg risankizumab, and placebo (i.e., withdrawal of risankizumab). Greater clinical remission and endoscopic response rates were observed for 360 mg risankizumab compared to placebo. Similar findings were observed for the 180 mg dose of Risankizumab; however there appeared to be a positive dose-response relationship for more rigorous secondary endpoints such as endoscopic and deep remission. Risankizumab, like other biologics, appears to be less effective among those with prior biologic failures. Safety was similar between all treatment groups with no dose-dependent observations.
Caution
There were relatively high rates of clinical remission among patients in the placebo (i.e., withdrawal) group, suggesting prolonged pharmacodynamic effects from intravenous induction risankizumab. Therefore, study results may underestimate the efficacy of risankizumab compared to placebo. Additionally, endpoints were not stratified by Crohn’s disease location or phenotype, so it remains unclear if risankizumab is similarly effective for small bowel, stricturing, or penetrating disease.
My Practice
So far, I am typically utilizing risankizumab for my patients with moderate-to-severe Crohn’s disease with prior biologic failures, including ustekinumab, vedolizumab, and anti-tumor necrosis factor agents. It was only approved by the FDA for use in Crohn’s disease about 6 months ago, in the summer of 2022. It is unknown if selective IL-23 inhibition performs superiorly to IL-12/23 inhibition. However, I have observed a clinical response to risankizumab among patients with prior loss of response ustekinumab. In the absence of adequate safety data, I do not yet recommend the use of risankizumab during pregnancy. In addition, this agent works very well in patients with concurrent psoriasis and we have appreciated a slight preference over ustekinumab from our dermatology colleagues.
For Future Research
Clinical predictors of response and failure of risankizumab therapy for Crohn’s disease are largely unknown. Future observational research should attempt to assess the performance of risankizumab among patients with specific disease phenotypes, such as small bowel fistulizing disease, perianal disease, and stricturing disease. With a growing selection of biologic mechanisms, comparative effectiveness research is also needed to help position risankizumab relative to other agents in the treatment algorithm for moderate-to-severe Crohn’s disease. The ongoing SEQUENCE trial will compare ustekinumab vs risankizumab in Crohn’s disease.
Conflicts of Interest
Dr. Dalal has received grant support from Janssen Pharmaceuticals and Pfizer Pharmaceuticals and has served as a consultant for Centaur Labs. Dr. Allegretti has received grant support from Janssen Pharmaceuticals, Pfizer Pharmaceuticals, and Merck Pharmaceuticals, and has served as a consultant for Janssen Pharmaceuticals, Pfizer Pharmaceuticals, AbbVie Pharmaceuticals, Ferring Pharmaceuticals, Merck Pharmaceuticals, Bristol Myers Squibb, Seres Therapeutics, Finch Therapeutics, Iterative Scopes, and Takeda Pharmaceuticals. Dr. Allegretti reports no conflicts.
@RPanaccione
@EdwardLoftus2
@IBDMD (David Rubin)
REFERENCES
- Eken A, Singh AK, Oukka M. Interleukin 23 in Crohn’s disease. Inflamm Bowel Dis 2014;20(3):587-95.
- Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 2008;57(12):1682-9.
- Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med 2016;375(20):1946-1960. doi:10.1056/NEJMoa1602773
- D’Haens G, Panaccione R, Baert F, et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet May 28 2022;399(10340):2015-2030.
Download the Article Summary (PDF)

