In Case You Missed It
Is Terlipressin for the Treatment of Hepatorenal Syndrome Ready for Primetime: Results of the CONFIRM Trial
 Sonali Paul, MD, MS
Sonali Paul, MD, MS
1Assistant Professor of Medicine, Division of Gastroenterology, Hepatology & Nutrition, Pritzker School of Medicine, the University of Chicago, Chicago, IL
This summary reviews Wong F, Pappas SC, Curry MP, et al. Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome. N Engl J Med. 2021 Mar 4;384(9):818-828.
Access the article through PubMed
Correspondence to Sonali Paul, MD, MS. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Is terlipressin (Terlivaz; Mallinckrodt Pharmaceuticals, UK), a vasoconstrictor of splanchnic and peripheral vasculature, safe and effective in Type 1 hepatorenal syndrome (HRS-1)?
Design: A Phase 3 randomized, double-blind, placebo-controlled trial of 300 patients with HRS-1 randomized in a 2:1 ratio to receive terlipressin or placebo for up to 14 days.
Setting: The trial was conducted across 60 sites in the United States and Canada from July 2016 through July 2019.
Patients: The study included 300 patients with HRS-1, cirrhosis, ascites, and rapidly progressive kidney failure (doublings of serum creatinine level to at least 2.25 mg/dL) within 14 days; 199 patients were assigned to the terlipressin group and 101 to the placebo group. Patients were excluded if their creatinine level had a sustained reduction of more than 20% (or a decrease below 2.25 mg/dL) within 48 hours of diuretic withdrawal and albumin infusions. Patients on midodrine and octreotide (current standard of care treatment for HRS-1) were eligible if both were discontinued prior to randomization. Other exclusion criteria included: serum creatinine level > 7.0 mg/dL, one or more large volume paracentesis (4 L within 2 days of randomization), sepsis or uncontrolled infection, cardiovascular disease, or initiation of renal replacement therapy (RRT) within 4 weeks. RRT includes hemodialysis, peritoneal dialysis, and other hemofiltration techniques to replace usual functions of kidneys.
Interventions/Exposure: Patients were randomly assigned in a 2:1 ratio to receive terlipressin (1 mg intravenous [IV] over 2 minutes every 5.5-6.5 hours) plus albumin (1 gram / kg to maximum of 100 grams on day 1, and 20-40 g/day daily after) or placebo plus albumin. In both groups, there were more males, the majority had alcohol associated liver disease as the cause of their cirrhosis (67% in terlipressin, 66% in placebo), with similar average model for end stage liver disease (MELD) score of 33. Study participation could be stopped for several reasons: (1) day 4 creatinine was greater than or equal to their baseline, (2) RRT initiation, (3) vasopressors started, or (4) underwent liver transplant or TIPS procedure.
Outcome: The primary end point was verified HRS reversal (defined in this study as 2 consecutive serum creatinine measurements of 1.5 mg/dL or less at least 2 hours apart) and survival without renal-replacement therapy for at least 10 days after treatment completion.
Secondary endpoints included: (1) HRS reversal (defined as serum creatinine < 1.5 mg/dL), (2) durability of HRS reversal (defined as HRS reversal without RRT to day 30), (3) HRS reversal among patients with systemic inflammatory response syndrome, and (4) verified reversal of HRS without recurrence of HRS by day 30.
Data Analysis: Intention-to-treat analysis.
Funding: Mallinckrodt Pharmaceuticals, manufacturer of terlipressin.
Results: More patients in the terlipressin group than placebo had verified HRS reversal (as defined above; 32% (n=63) vs 17% (n=17); P=0.006 respectively). The secondary endpoints also had greater success in the terlipressin group as compared to placebo (Table 1). Transplantation free survival up to 90 days did not differ significantly between the groups. However, there were more adverse events in the terlipressin group than placebo including abdominal pain, nausea, diarrhea, and respiratory failure. Notably, death from respiratory complications occurred more often in the terlipressin group (n=22, 11%) versus placebo (n=2, 2%).
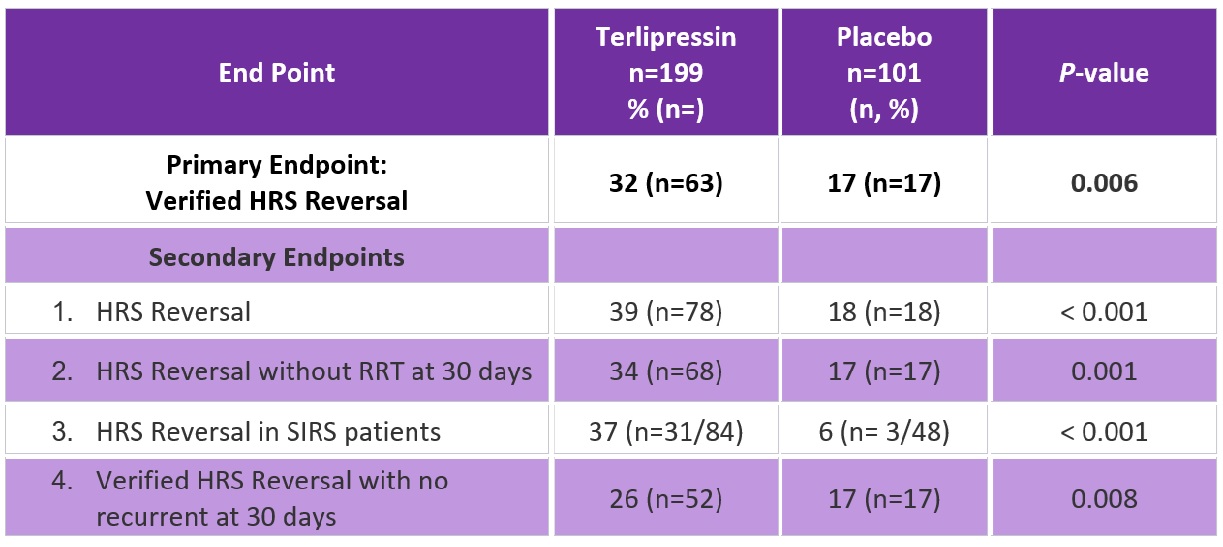
Abbreviations: HRS, hepatorenal syndrome; RRT, renal replacement therapy; SIRS, systemic inflammatory response syndrome.
COMMENTARY
Why Is This Important?
Patients with decompensated cirrhosis and ascites are at risk for HRS-1, characterized by rapidly progressive kidney failure with high mortality rates in the absence of liver transplantation. Vasoconstrictors to increase mean arterial pressure have been used to combat the systemic vasodilation that is one of the main mechanisms leading to HRS. Although midodrine, an orally active vasoconstrictor, combined with octreotide is standard treatment in the US, it has limited efficacy.1 Given the high mortality of HRS, effective treatments are needed.
Terlipressin, a synthetic vasopressin that constricts the splanchnic and systemic vasculature, has shown success in HRS treatment in Europe, but was not available in the US until September 2022 when the FDA approved it for treatment of HRS based largely on this randomly controlled trial and other parts of the world. However, side effects due to vasoconstriction, including abdominal pain or ischemia of fingers, skin, etc, as well as pulmonary edema when combined with albumin are common.1
More patients in the terlipressin group than placebo had verified HRS reversal (as defined above; 32% (n=63) vs 17% (n=17); P=0.006 respectively). Death from respiratory complications occurred more often in the terlipressin group (n=22, 11%) versus placebo (n=2, 2%).
Caution
Despite terlipressin improving HRS-1 in more patients than placebo, respiratory failure and death from respiratory failure also occurred in more patients receiving terlipressin.
Notably, the definition of HRS(creatinine > 2.25 mg/dL) in the trial does not follow the consensus definition of HRS which is an increase in the creatinine level of > 0.3 mg/dL from baseline that does not improve with volume expansion. If the consensus definition had been used, it is possible that terlipressin would show a larger response, as HRS reverses at lower creatinine levels.
My Practice
Any new or worsening renal insufficiency is worrisome in a patient with decompensated cirrhosis. A thorough work up ruling out other causes, discontinuation of diuretics, and initiation of volume expansion is required. HRS is diagnosed when there is no improvement in the creatinine after volume expansion. Standard treatment in the United States is midodrine, octreotide, and albumin in addition to a prompt liver transplant evaluation. If there is no improvement within 48 hours, I will transfer these patients to the intensive care unit for a trial of vasoactives, primarily norepinephrine, which has demonstrated similar efficacy to terlipressin although there is less data.1 Given the significant cardiopulmonary complications associated with terlipressin, I do not currently recommend routine use and I individualize use on a case-by-case basis.
The question of renal replacement therapy is often brought up. In my practice, if they are a viable liver transplant candidate or if their MELD is being driven mainly by the creatinine (and bilirubin and INR are fairly preserved / improving), I will pursue this. However, in those that are not liver transplant candidates, it often becomes a bridge to nowhere (especially if unable to tolerate hemodialysis) and I often then focus on goals of care.
For Future Research
Future research and safety data is needed before universal use of terlipressin for HRS. Future studies could consider using the current consensus definition of HRS, as there may be a benefit in certain patient populations (perhaps lower creatinine levels indicating less sick patients).
Conflict of Interest
Dr. Paul has no relevant conflicts of interest.
REFERENCE:
- Biggins S, Angeli P, Garcia-Tsao G, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;74: 1014-48.

