What is esophageal physiologic testing?
Physiologic testing of the esophagus (food-pipe) includes a variety of radiologic, endoscopic, motility, reflux tests that gastroenterologists use to evaluate the physiology (normal function) of the esophagus. Radiologic tests are performed using X-rays. Endoscopy utilizes a camera to evaluate the inside of the food-pipe. Motility testing evaluates the function of the food-pipe which is essential to move food from the mouth to the stomach. Gastroesophageal reflux testing aims to quantify and assess the amount of reflux that a patient is experiencing over a period of time and the association between the reflux episodes and symptoms that the patient experiences.
Diagnostics to evaluate Motility (movement) disorders include:
- Upper endoscopy
- Barium esophagram
- Esophageal manometry
- EndoFLIP planimetry
Diagnostics to evaluate reflux related symptoms include:
- Catheter passed 24-hour combined multichannel intraluminal impedance-pH monitoring
- Wireless esophageal capsule based pH monitoring (Bravo pH)
Why might a patient need esophageal physiologic testing?
Patients who experience symptoms such as heartburn, trouble with swallowing, chest pain (that is unrelated to heart disease) might require physiologic testing to determine if a motility disorder is causing these problems or better manage GI disorders of the esophagus. While most patients with GERD are treated with acid suppression medication successfully without the need to quantify the amount of reflux they are experiencing, further testing is necessarily in the following settings:
- Reflux symptoms (like heartburn, regurgitation, chest pain) not adequately controlled with acid suppression medications (E.g. Omeprazole, pantoprazole etc.)
- Patients considering anti-reflux surgery such as fundoplication etc. to predict a patient’s reflux will respond to surgery
What are the various tests used for evaluation of motility disorders of the esophagus?
Upper endoscopy or an EGD (esophagogastroduodenoscopy)?
An upper endoscopy is usually the first step in evaluating symptoms such as trouble with swallowing, pain with swallowing, regurgitation of food/secretions, heartburn that is not going away with medication. Gastroenterologists perform an upper endoscopy that uses a light and camera on a long flexible scope to evaluate the food-pipe, stomach and the first part of the small intestine called the duodenum while the patient is under anesthesia. Upper endoscopy is not a test that typically evaluates esophageal function. However, it is used to :
- Look for inflammation of the esophagus due to reflux from the stomach into the esophagus, a type of inflammation called eosinophilic esophagitis, and infections in the esophagus
- Rule out obstruction in the esophagus due to a mass/narrowing (i.e. peptic stricture)
- Evaluate for precancerous changes that can occur in some patients with severe reflux disease (also known as Barrett’s esophagus).
- Perform physiologic procedures such as Endoscopic functional lumen imaging probe (EndoFLIP) or Bravo pH capsule attachment (discussed in detail below).
A full overview of the upper endoscopy is detailed in this webpage: https://gi.org/topics/upper-gi-endoscopy-egd/
Barium esophagram
Barium esophagram (or Barium swallow) is a non-invasive radiologic procedure which is sometimes used to evaluate the transfer of liquid Barium and a Barium tablet through the esophagus into the stomach. During this procedure, the patient is asking to swallow 100-250 ml of Barium liquid and X-rays are taken to watch the barium pass through the stomach. A barium tablet (which is about 13 mm) is also administered to patient to see if it gets stuck anywhere along the esophagus. The esophagus moves the food/drink we swallow in a coordinated fashion down into the stomach and this movement is called peristalsis. This study provides information regarding the quality of peristalsis in that moves the Barium, any location where the tablet might get stuck which might identify improper function of the sphincter muscles in the esophagus and identify the configuration of the stomach and esophagus in patients who have previously undergone surgery of their esophagus. Sometimes a “baseline” (before surgery) esophagram is compared to a repeat exam after a treatment of a motility disorder to follow response. Specific “timed protocol” esophagrams are sometimes performed where x-ray images are captured at timed intervals (1 min, 2 min and 5 min) to further quantify the delay in the emptying of the esophagus.Esophageal Manometry
Esophageal manometry is the gold standard test to evaluate the motility (movement) of the food-pipe in patients who have symptoms suspicious for a motility disorder. Manometry is also performed in patients considering surgery to treat GERD, to make sure that their esophagus is working properly to tolerate the surgery. Finally, esophageal manometry is used to accurately find the location of the lower esophageal sphincter (LES) for proper placement of a reflux catheter that evaluates GERD (see diagnostics to evaluate GERD below).
- What is a motility disorder?
Normal esophageal function involves coordinated peristalsis that moves food down to the stomach. The lower esophageal sphincter is a muscle located at the junction between the stomach and the esophagus and has to relax appropriately for the food to enter the stomach. Motility disorders can occur if normal peristalsis is disrupted or if the relaxation of the LES is impaired thereby causing food to get stuck or stay in the esophagus for too long. These disorders can cause symptoms such as difficulty swallowing, chest discomfort, regurgitation of food/liquid that is unable to pass into the stomach, and heartburn that is not controlled by acid suppression medication.
- How is an esophageal manometry performed?
The esophageal manometry is performed by a trained technician who will first anesthetize one of the patient’s nostrils with a local numbing medication. A long flexible motility catheter (tube) is then slowly advanced through the nose into the food-pipe and the stomach of the patient while sitting upright. Although this can cause gagging in some patients, the technician will instruct the patient regarding swallowing and breathing maneuvers to help ease this sensation. Once the catheter is successfully placed in position, the patient is asked to lie down. The technician will then start recording the pressures in the esophagus while patient is not swallowing. Then the patient is instructed to swallow upon request during “test swallows” where the technician gives a specific amount of water (which is usually salty). The catheter in the food-pipe has sensors along it that measure the pressure generated by the esophagus as well as the contraction of the sphincter muscles of the esophagus. Some swallows might also be performed while patient sits in an upright position The technician might also do some other maneuvers such as swallows in rapid succession or drinking from a cup using a straw. Once the pressure measurements are recorded in the computer connected to the manometry catheter, the catheter is removed from the patient’s nose.
- What are the potential side-effects of esophageal manometry?
Most patients tolerate esophageal manometry without pain although they can experience some discomfort due to gag reflex briefly as the catheter passes into the throat. Minor side effects include throat pain/discomfort that lasts beyond the duration of the procedure, nose bleed, retching, gagging and rarely sinus congestion. Improper placement of the catheter into the wind pipe instead of the food pipe can cause cough and sensation of choking which will cause the technician to remove the catheter immediately.
- How does a patient prepare for esophageal manometry?
Patients will be asked to not eat or drink anything after midnight prior to the procedure (or at least 6-8 hrs prior) to ensure that anything in the stomach has emptied out into the intestines. This reduces the risk of causing any food/drink to come up from the stomach during the procedure and going into the lungs (also known as aspiration). Your gastroenterologist will instruct you regarding any medications should be held and for how long prior to the procedure. Examples include certain pain medications like morphine, codeine, oxycodone etc. and medications used for anxiety/sleep-aids such as Valium etc. Patient might also be instructed to hold blood thinning medications such as Coumadin, Eliquis, Plavix etc. Dosing of insulin in patients with diabetes also needs to be adjusted sometimes due to fasting prior to the procedure. After the completion of the procedure, patient is immediately able to eat/drink and return to normal activities.
EndoFLIP planimetry
During an upper endoscopy, gastroenterologists will sometimes use a balloon catheter called EndoFLIP to measure the distensibility (a measure of stretch) and also the cross sectional area of the junction between the stomach and the esophagus (or GE junction). EndoFLIP involves passing a deflated balloon catheter during upper endoscopy while the patient is under anesthesia. The endoscope is used to visualize appropriate placement of the EndoFLIP into the stomach. The balloon on the catheter is then slowly inflated incrementally typically to 60 ml at which points measurements are taken. The balloon catheter is then deflated and removed.
- When is EndoFLIP performed?
EndoFLIP is primarily used to provide additional information regarding some motility disorders that cause a delay in passage of food/drink through the GE junction. However, when an esophageal manometry is unable to be performed due to difficulty in passing the motility catheter into the stomach, EndoFLIP can also be used to diagnose certain motility disorders. Lastly, EndoFLIP can be used during and after some treatment procedures such as peroral endoscopic myotomy which disrupts the muscle in and around the GE junction to allow for better passage of food etc. In this setting, optimal treatment response can be ascertained by comparing measurements before the treatment to those after the treatment.
- How does a patient prepare for EndoFLIP?
As EndoFLIP is performed only during upper endoscopy, preparation for it is the same as for an upper endoscopy. In addition, patients may be instructed to hold medications that may interfere with motility of the esophagus such as narcotics, benzodiazepines a few days prior to the study. Patients are under sedation while they undergo EndoFLIP.
- What are potential side-effects of EndoFLIP procedure?
Patients are under anesthesia when EndoFLIP is performed. The balloon catheter utilized Endoflip measurements is soft and flexible with pressure sensors that alarm if the catheter detects any high pressure as a safety feature. Therefore, patients typically do not complain of any side effects due to the FLIP procedure.
What are the tests used for evaluation of gastroesophageal reflux disease?
Reflux testing can be performed using two different modalities: wireless capsule used to detect the change in pH of the esophagus vs. catheter based pH-impedance testing which are described below. Both of these tests occur while patient is at home carrying out their typical activities, eating their usual diet to mimic their day-to-day lifestyle as much as possible.
Most commonly, patients are instructed to stop acid suppression medicines such as proton pump inhibitors (e.g. pantoprazole, omeprazole, esomeprazole, etc.) about 1 week prior to the test and histamine-2 blockers (e.g. Famotidine, ranitidine etc.) for up to 3 days prior to the test. However, the studies are sometimes performed while on these medications as determined by the gastroenterologist.
Wireless esophageal capsule based pH monitoring
Esophageal pH monitoring provides objective data regarding the exposure of the esophageal lining to acid coming from the stomach. A small wireless capsule (also known as Bravo capsule) is attached to the lining of the esophagus during an upper endoscopy using a flexible plastic catheter (Figure 1) that is placed into the patient’s mouth and advanced into the esophagus. This is done while patient is under anesthesia. After attaching the capsule in the esophagus, the catheter is removed. The attached capsule (Figure 2) contains a pH sensor and transmits data from inside the esophagus to a wireless recorder that patient wears on a belt outside the body. Once patient recovers from anesthesia, he is instructed to resume their normal activities and to record the times when he starts and stops a meal, lies down and arises from a sleeping position. The patient is also asked to record the time when they experience any reflux symptoms by pushing specific buttons on the wireless recorder and also by writing in a diary. The recording typically lasts for 48 hours although in some cases it can continue for longer periods of time (72-96 hrs). After the specified study period, the patient returns the wireless recorder and their diary. The capsule attached to the esophagus falls off on its own and passes out of the patient in their stool in a few days. The data recorded in the wireless recorder is downloaded and evaluated by a physician to quantify the amount of time that the esophagus is exposed to gastric acid as well as to correlate symptoms of GERD with timing of when their reflux occurs. As there is no catheter that’s in the patient’s nose, patients are able to tolerate recording of their reflux symptoms for longer periods of time (2-4 days). https://gi.org/wp-content/uploads/2021/06/Bravo-Catheter-Esophagus.jpg
Figure 1: Bravo catheter that deploys the capsule to attach to lining of the esophagus

Figure 2: Wireless pH capsule that stays in the esophagus

Figure 3: Wireless pH capsule recorder (worn by patient on their belt
- How does a patient prepare for wireless esophageal pH monitoring?
As esophageal pH capsule is attached during upper endoscopy, preparation for it is the same as for an upper endoscopy. In addition, patients are usually instructed to hold acid suppression medications a few days prior to the study.
- What are the potential side-effects/limitations of wireless pH capsule based reflux monitoring?
An important limitation during this procedure is that the patient cannot undergo an MRI scan during the time that the pH monitoring capsule is attached to the esophagus. Also, patients who have implanted devices such as pacemakers, defibrillators etc. and those that have abnormalities of the esophagus such as narrowing in the esophagus (strictures) or varices (varicose veins of the esophagus that can easily cause severe bleeding) cannot undergo this test. Occasionally, patients undergoing wireless pH capsule monitoring can experience a sensation of discomfort with swallowing or chest pain at the site where the capsule is attached. Rarely, if patient is unable to tolerate these symptoms, the capsule is removed with repeating upper endoscopy. Patients who have a nickel allergy should not undergo this procedure as the capsule contains nickel.
24-hour combined multichannel intraluminal impedance-pH monitoring
In contrast to the esophageal pH monitoring capsule, combined multichannel intraluminal impedance-pH (MII-pH) monitoring utilizes a very thin catheter that has sensors to detect reflux of any liquid from the stomach that backs up into the esophagus. This catheter is passed into the patient's nostril (after numbing the nostril with a local anesthetic) and advanced into the lower portion of the esophagus (Figure 4). The appropriate location where the tip of the catheter should be placed is determined by measurement of the LES using esophageal manometry (see description of manometry) detects where the lower esophageal sphincter is present. The other end of the catheter that leaves the patient nostril is connected to a battery operated recorder that is worn by the patient for 24 hours. The catheter detects not acid, weakly acid or nonacid reflux using impedance measurements. During the 24-hour study, the patient records any reflux symptoms they experienced in the diary and by pushing buttons on the recorder. They also record times of their meals and sleeping (when they are laying down on their back). After 24-hour study period is completed, the patient returns the recorder and diary, and the catheter is removed from the patient's nostril. The data recorded is then downloaded and interpreted by the physician to evaluate the amount and type of reflux as well as correlate symptoms to reflux episodes. While this test records for only 24-hour period, it may be more sensitive in detecting gastroesophageal reflux because of the ability to detect movement of liquid into the esophagus regardless of the pH level.
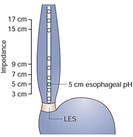
- How does a patient prepare for 24-hr MII-pH monitoring?
Patients typically fast after midnight (or at least for 6 hours prior to this test) as they will commonly have manometry just before pH impedance catheter placement. In addition, patients may be instructed to hold acid suppression medications and blood thinner medications a few days prior to the study.
- What are potential side-effects of 24-hr MII-pH monitoring procedure?
24-hr MII-pH monitoring is typically very well-tolerated by patient's due to the thin diameter of the catheter placed in the nose. Most patients get used to having the catheter in their throat within a few minutes of the study. Although some patients feel mild throat discomfort while the catheter is in place for the duration of study, this discomfort is rarely significant enough for patients to request removal of the catheter. Nose bleed or sinus infections are uncommon with this procedure due to the thin catheter.
References
Gyawali, C. Prakash MD, MRCP, FACG1; Carlson, Dustin A. MD2; Chen, Joan W. MD3; Patel, Amit MD4; Wong, Robert J. MD, MS, FACG (GRADE Methodologist)5; Yadlapati, Rena H. MD, MSHS6 ACG Clinical Guidelines: Clinical Use of Esophageal Physiologic Testing, The American Journal of Gastroenterology: September 2020 - Volume 115 - Issue 9 - p 1412-1428. doi: 10.14309/ajg.0000000000000734.
Radu Tutuian, MD and Donald O. Castell MD. Gastroesophageal reflux monitoring: pH and impedance. GI Motility online (2006) doi:10.1038/gimo31.Author(s) and Publication(s)
Sravanya Gavini, MD, MPH, UT Southwestern Medical Center – Updated April 2021.

