Linaclotide Effective for Functional Constipation in 6-17 Year Olds: First FDA-Approved Constipation Treatment for Children
 Ahmad Abu-Heija, MBBS
Ahmad Abu-Heija, MBBS
Consultant Gastroenterologist, Oak Ridge Gastroenterology Associates, Oak Ridge, TN.
This summary reviews Di Lorenzo C, Khlevner J, Rodriguez-Araujo G, et al. Efficacy and safety of linaclotide in treating functional constipation in paediatric patients: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Gastroenterol Hepatol 2024;9(3):238-250.
Access the article through PubMed
Correspondence to Ahmad Abu-Heija, MBBS, Associate Editor. Email: EBGI@gi.org
Keywords: constipation, diarrhea, double-blind method, RCT, treatment outcome.
STRUCTURED ABSTRACT
Question: Is linaclotide, a guanylate cyclase C receptor agonist approved for treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation (IBS-C) in adults, effective, safe, and well-tolerated for treatment of bothersome functional constipation symptoms in pediatric patients (ages 6-17 years)?
Design: Randomized, double-blinded, placebo-controlled multicenter, parallel-group, 12-week, phase 3 trial.
Setting: Sixty-four clinic or hospital sites in 7 countries, including United States, Canada, Israel, Italy, and the Netherlands.
Patients: Study patients must have had ≤2 unassisted defecations per week for ≥2 months before the screening visit and meet a modified Rome III criteria for pediatric functional constipation (Table 1). Patients with IBS were excluded from this study.
Intervention: linaclotide 72 µg oral once daily taken 30 minutes before a meal at the same time each day vs identical placebo for 12 weeks.
Outcomes: Primary efficacy endpoint was change from baseline (CFB) in spontaneous bowel movements (SBM) frequency rate (SBMs per week) over the study period. SBM was defined as a bowel movement that occurred in the absence of a rescue medication (e.g., laxative, enema, etc.) the day of or before the movement. The secondary efficacy endpoint was CFB in stool consistency using the pediatric Bristol Stool Form Scale. Additional endpoints were CFB in 12-week frequency rate of complete SBM, straining with bowel movements, overall responder (defined as >2 SBM/week increase from baseline), and abdominal bloating among others.
Data Analysis: Modified intention-to-treat analysis, including all patients who received at least 1 dose of the study intervention, using ANCOVA analysis to identify differences in primary and secondary endpoints between linaclotide and placebo. Change from baseline (CFB) reported as least-squares mean (LSM). Pre-specified analyses also stratified results by age group: 6-11 years old and 12-17 years old.
Funding: Ironwood Pharmaceuticals and AbbVie Pharmaceuticals, manufacturer of linaclotide. The funders of the study participated in the study design, research, data collection, data analysis, data interpretation, writing of the article, and approval of submission for publication.
Results: Among 1,002 patients screened and 330 met inclusion criteria and were randomly assigned between October 2019 and March 2022. Overall, median age was 11.0 years, 55% were female, and 70% were White. Baseline SBMs per week were 1.3 per week in the placebo group and 1.2 per week in the linaclotide group.
Compared to the placebo group, linaclotide-treated patients had significantly greater improvements in SBM per week. Specifically, linaclotide-treated patients increased from 1.2 SBM per week to 3.4 SBM per week while placebo-treated patients only increased from 1.3 SBM per week to 2.3 SBM per week (Figure 1). Based on LSM change from baseline, linaclotide-treated patients also demonstrated significant improvements in stool consistency, straining, and frequency of complete SBM per week. Linaclotide-treated patients were also more likely to be overall responders with an increase of > 2 SBM per week: 43% vs 23%, P = 0.0001. Improvement in SBM per week was numerically higher in the linaclotide-treated 6-11 year old group vs the linaclotide-treated 12-17 year old group, although this increase did not achieve statistical significance.
Numerically, a lower number of patients used rescue drugs in the linaclotide group, but this was not statistically significant and was above 50% in both arms of the study. Incidence of diarrhea was numerically higher in linaclotide-treated group vs placebo-treated group: 4% vs 2%.
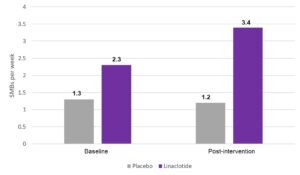
Figure 1. Primary endpoint results in increase in spontaneous bowel movements (SBM) per week.

Table 1. Modified Rome III criteria for pediatric functional constipation.
COMMENTARY
Why Is This Important?
Constipation is one of the most common functional gastrointestinal disorders in pediatric patients, affecting approximately 1 in 7 of children worldwide, with significant impact on quality of life.1 It’s also one of the most common referrals for our pediatric gastroenterology colleagues. Nonpharmacologic therapies, including parental education, behavioral modifications (e.g., regular toileting for 5-10 minutes after meals), and diet modification with fiber supplementation or addition of kiwifruit, prunes, or prune juice, are the first line of treatment. Although glycerin and bisacodyl suppositories are approved for use in children >6 years old with constipation, no other over-the-counter treatments appear to be officially Food and Drug Administration (FDA)-approved for pediatric constipation. Nevertheless, polyethylene glycol (MiraLax; Bayer US, Whippany, NJ) is frequently recommended for pediatric constipation2 based on randomized controlled trial data, although constipation persists in more than 20% of patients necessitating other interventions. Unfortunately, among prescriptions treatments for adult chronic idiopathic constipation, neither prucalopride nor lubiprostone have been shown to be superior to placebo in children.3-4 Given the lack of proven constipation treatments for pediatric patients and the frequency of this complaint, new treatments are needed.5
Linaclotide is a guanylate cyclase C agonist approved for treatment of chronic idiopathic constipation (72ucg or 145ucg) and IBS-C (290ucg) in adults. Results from this study led to FDA approval of linaclotide as the first approved prescription treatment for pediatric (ages 6-17 years) patients with functional constipation.
Key Study Finding
In this double-blinded, randomized, placebo-controlled trial linaclotide improved SBMs per week over baseline significantly.
Patients also had a relatively rapid benefit from linaclotide with 57% of patients having an SBM within 48 hours of treatment.
Caution
This study only followed patients for 12 weeks, which is a relatively brief period given the chronicity of functional constipation and as such long-term effects cannot be analyzed. In addition, rescue medications were used frequently by placebo-treated patients (60%) and linaclotide-treated patients (52%).
My Practice
Based on consultation with pediatric gastroenterology colleagues, linaclotide will be incorporated into their management of pediatric functional constipation, but will be reserved for patients that first fail non-pharmacologic interventions and polyethylene glycol. While the 72ucg dose will be used for 6-11 year olds, the 145ucg dose may be used in older adolescents, especially if they don’t get an adequate response to the 72ucg dose.
For Future Research
Long-term real-world data will be helpful in confirming safety and efficacy of linaclotide in children. Further sub-group analysis based on gender, type of previous failed treatments, and age will be beneficial.
Conflict of Interest
Dr. Abu-Heija reports no potential conflicts of interest for this summary.
REFERENCES
- Lewis ML, Palsson OS, Whitehead WE, et al. Prevalence of functional gastrointestinal disorders in children and adolescents. J Pediatr 2016; 177:39-43.e3.
- Gordon M, MacDonald JK, Parker CE, et al. Osmotic and stimulant laxatives for the management of childhood constipation. Cochrane Database Syst Rev 2016(8):CD009118.
- Mugie SM, Korczowski B, Bodi P, et al. Prucalopride is no more effective than placebo for children with functional constipation. Gastroenterology 2014;147:1285–95.
- Benninga MA, Hussain SZ, Sood MR, et al. Lubiprostone for pediatric functional constipation: randomized, controlled, double-blind study with long-term extension. Clin Gastroenterol Hepatol 2022;20:602–10.
- Salvatore S. Linaclotide for paediatric functional constipation. Lancet Gastroenterol Hepatol 2024;(9)3: 191-92.

