PROFILE: Can Molecular Biomarkers Predict Outcomes to Crohn’s Disease Treatment?

Bharati Kochar, MD, MS
Assistant Professor of Medicine, Division of Gastroenterology, Massachusetts General Hospital; Investigator, The Mongan Institute, Harvard Medical School, Boston, MA
This summary reviews Noor NM, Lee JC, Bond S, et al. A biomarker-stratified comparison of top-down versus accelerated step-up treatment strategies for patients with newly diagnosed Crohn’s disease (PROFILE): a multicentre, open-label randomised controlled trial. Lancet Gastroenterol Hepatol 2024; 9: 415-27.
Access the article through PubMed
Keywords: Crohn’s disease, biomarkers, biologics
Correspondence to Bharati Kochar, MD, MS. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Can a prognostic molecular biomarker, derived from T-cell transcriptional signatures, help determine who will benefit from an early top-down versus accelerated step-up treatment strategy for adults with newly diagnosed Crohn’s disease (CD)?
Design: The PROFILE (predicting outcomes for CD using a molecular biomarker) study is a multi-centered, open-label, biomarker-stratified randomized controlled trial (RCT).
Setting: Patient were screened from 40 hospitals in the United Kingdom. The study enrolled from December 2017 to January 2022.
Patients: This study included patients aged 16–80 years old with symptomatically active (Harvey-Bradshaw Index [HBI] >7) CD diagnosed within 6 months of screening. To be eligible, subjects had to have biochemical evidence of active inflammation, defined as a C-reactive protein above the upper limit of normal and/or a fecal calprotectin >200 ug/g. Additionally, subjects were required to have endoscopically active disease, defined as Simple Endoscopic Score for CD ≥4, and be naïve to immunosuppressive therapies. Patients with “clinically significant” obstructive or perianal disease were excluded.
Interventions/Exposure: PredictSURE-IBD (PredictImmune Ltd, Cambridge, UK) is a T-cell transcriptional signature that was intended to help determine which patients with CD may benefit from upfront biologic therapy. Based on results of this blood-based test, patients were identified as high risk of inflammatory bowel disease (IBD) treatment escalation (IBDhi) versus those who were at low risk for IBD treatment escalation (IBDlo).
Using stratified block randomization based on biomarker group (IBDhi vs IBDlo), disease location (colonic vs other) and mucosal inflammation (mild vs moderate vs severe), eligible participants were then randomized 1:1 to a top-down or accelerated step-up treatment (Figure 1).
Outcomes: The primary endpoint was the incidence of sustained steroid-free and surgery-free remission from the completion of the initial steroid induction to week 48. Objective evidence of disease, such as elevated inflammatory markers, were required to determine failure. There were 5 secondary end points, which are as follows: (1) endoscopic remission by week 48, (2) quality of life measured by the IBD-Q, (3) number of flares requiring treatment escalation, (4) cumulative steroid exposure and (5) number of Crohn’s-related hospitalizations and surgeries.
Data Analysis: The primary analysis was to determine the interaction between the intervention (PredictSURE-IBD) and treatment to determine if the primary outcome can be achieved. The sample size was determined for 92% power with a 2-sided 5% P-value. The primary analysis was an intention to treat analysis, but a secondary per-protocol analysis was also conducted.
Funding: Funding for this trial was provided by Wellcome and PredictImmune Ltd, which are the commercial entities with stake in the biomarker studied.
Results: Among 386 newly diagnosed CD patients enrolled in the trial, mean age was 33-34 years old, female sex was 46%-47%, White ethnicity was 87%-89%, ileal disease alone was 33%-34%, colonic disease was 26%-28%, and remainder was ileocolonic disease. Disease behavior was classified as inflammatory in 85%-88%, stricturing in 11%-14%, and penetrating in 1%. Biomarker status (IBDhi vs IBDlo) was evenly split at 50% in step-up arm and top-down treatment arm.
Sustained steroid-free and surgery-free remission was more frequent in the top-down treatment arm than the accelerated step-up arm: 79% vs 15%, P< 0.0001 (Figure 2). However, there was no significant biomarker-treatment interaction, meaning that the biomarker was not useful for guiding therapy for CD. Additionally, all secondary outcomes were better in the top-down group than the step-up ground, but again the biomarker did not exert an influence on the outcome. Endoscopic remission, defined as absence of ulceration at week 48, was also significantly higher in the top-down treatment arm: 67% vs 44%, P< 0.0001. They reported 11 urgent abdominal surgeries in the trial period (2 in the top-down group and 9 in the step-up group).
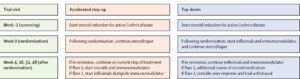
Figure 1. Trial visits and escalation summary.
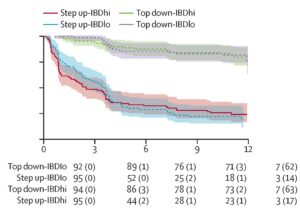
Figure 2. Kaplan-Meier analysis of time to flare, surgery, or both. Time to first event by biomarker–treatment group with data censored at 12 months.
COMMENTARY
Why Is This Important?
This was a negative study because the PredictSURE-IBD biomarker did not predict which patients would benefit from step-up vs top-down therapy for CD. However, it is an important study because it provides strong evidence that early (<6 months of diagnosis) initiation of top-down treatment with biologic therapy for CD is critical. The clinical response for CD patients treated early with infliximab and an immunomodulator was 79% with endoscopic remission of 67%, which is much higher than many CD trials.
The first trial to study this concept was the REACT trial, an open-labeled cluster randomized trial in Belgium and Canada.1 They randomized centers to “conventional management” which during the study period (2010-2013) was step-up treatment or early combined immunosuppression and assessed steroid-free remission based on a HBI at 12 months. As a secondary analysis, they determined major adverse events (surgery, hospitalizations, other disease-related complications) at 24 months. The primary outcome in this trial was also negative, but they demonstrated a lower rate of major adverse events in the early combined immunosuppression arm. The REACT trial enrolled patients with a median CD duration between 12-13 years, which is much later in the disease course than patients in the PROFILE study. A recent meta-analysis of 25 trials testing biological agents for the treatment of IBD demonstrated that induction of remission was more successful in CD when the drug was started in patients with ≤18 months disease duration compared with those with disease duration >18 months, while this stratification by disease duration was not noted for patients with ulcerative colitis.2 The difference in disease duration of patients enrolled in the REACT and PROFILE trials may be a leading explanation for the difference noted. Additionally, patients in the PROFILE study had a higher mean HBI score (9-10) compared with patients in the REACT trial (4). Another possible explanation may be that PROFILE used objective evidence of remission in addition to a symptomatic measure.
CD is often a transmural disease. Delays in effectively treating CD may lead to disease complications such as medically refractory disease, stricturing or penetrating complications. The robust inflammation that characterizes CD requires early and up-front biologic therapy. Ultimately, this emphasizes the importance of avoiding recurrent courses of steroids for patients with CD and willingness to start some type of biologic therapy earlier in disease course.
The importance of biomarkers in assessing IBD is increasingly recognized. The CALM study randomized patients with CD naïve to immunomodulators to monitoring with clinical symptoms alone or symptoms and biomarkers to monitor disease activity with this monitoring guiding treatment decision making.3 They concluded that a significantly higher proportion of patients in the tight control group achieved the primary endpoint of mucosal healing at week 48. Trials like CALM and PROFILE highlight the importance of studying treatment strategies for IBD. Although the PredictSURE-IBD panel was not a predictive biomarker, a recent clinical practice guideline highlights the appropriate role of biomarkers for the management of CD.4
Key Study Findings
There was no significant biomarker-treatment interaction with PredictSURE-IBD, meaning that the biomarker was not useful for guiding step-up vs top-down therapy for CD within 6 months of diagnosis.
Caution
A major limitation of the PROFILE trial is that treating physicians were blinded to the intervention (biomarker result), but not to the treatment (step up versus top down) which may lead to an over estimation of the treatment effect in the top-down treatment group. Also, about one third of patients did not have an end of trial colonoscopy due to COVID-19 related shutdowns.
My Practice
While my use of biomarkers is not changed based on the PROFILE trial, this trial provides robust data to support upfront biologic therapy for Crohn’s disease with numbers to cite to patients for likelihood of success when a biologic is started within 6 months of diagnosis. Despite guideline recommendations that biologic agents are first line therapy for Crohn’s disease, there is a tremendous amount of hesitation from patients and sometimes providers to begin biologics early in the course of disease. Although caution about starting a longitudinal medication is understandable, this study provides reassurance that the best chance of success for controlling Crohn’s disease comes with early initiation of biologic therapy.
For Future Research
Unfortunately, in tertiary care practice, it is increasingly rare to see patients within 6 months of a new diagnosis of IBD. In the US, the next steps should be implementation research to facilitate early initiation of biologic therapy in appropriate patients and obtain a better understanding of obstacles both from a patient’s perspective as well as a provider’s perspective.
Conflict of Interest
Dr Kochar received consulting fees from Pfizer and Bristol Meyers Squibb.
REFERENCES
- Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 2015;386:1825-34.
- Ben-Horin S, Novack L, Mao R, et al. Efficacy of biologic drugs in short-duration versus long-duration inflammatory bowel disease: a systematic review and an individual-patient data meta-analysis of randomized controlled trials. Gastroenterology 2022;162:482-494.
- Colombel J-F, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017;390:2779-2789.
- Ananthakrishnan AN, Adler J, Chachu KA, et al. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn’s Disease. Gastroenterology 2023;165:1367-1399.

