Vedolizumab: An EARNEST Option for the Management of Chronic Pouchitis
 Kristin E. Burke, MD, MPH1,2 and Bharati Kochar, MD, MS 2,3
Kristin E. Burke, MD, MPH1,2 and Bharati Kochar, MD, MS 2,3
1Instructor, Division of Gastroenterology, Massachusetts General Hospital, Boston, MA
2Investigator, The Mongan Institute, Harvard Medical School, Boston, MA
3Assistant Professor of Medicine, Division of Gastroenterology, Massachusetts General Hospital, Boston, MA
This summary reviews Travis S, Silverberg MS, Danese S, et al. Vedolizumab for the treatment of chronic pouchitis. N Engl J Med 2023;388(13):1191-1200.
Access the article through PubMed
Correspondence to Bharati Kochar, MD, MS, Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Is vedolizumab a safe and effective treatment for patients with chronic pouchitis after ileal pouch anal anastomosis (IPAA) for ulcerative colitis (UC)?
Design: The EARNEST study is 34-week, multicenter, randomized, double-blind, placebo-controlled phase 4 trial. It is the first randomized controlled trial (RCT) to study the efficacy and safety of a biologic therapy in management of pouchitis after IPAA for UC.
Setting: Patients were recruited from 13 sites in North America and 18 sites in Europe.
Patients: Inclusion criteria were: (a) 18- to 80-year-old patients; (b) status post IPAA for UC at least 1 year prior to screening; (c) active chronic pouchitis defined as a modified pouchitis disease activity index (mPDAI)* score of at least 5 with a subscore of at least 2 on the endoscopic domain; and (d) at least 3 episodes of pouchitis within 1 year before the screening visit treated with at least 2 weeks of an antibiotic or other therapy, or continuous antibiotics for at least 4 weeks. Primary exclusion criterion was previous vedolizumab use.
Intervention: Vedolizumab 300 mg intravenous on day 1, and at weeks 2, 6, 14, 22, and 30. All patients received oral ciprofloxacin 500 mg twice daily from the time of randomization through week 4. Subsequently, antibiotics were not permitted from weeks 5-14. Additional antibiotics were permitted for pouchitis flares after week 14. Oral corticosteroids were also permitted throughout the trial if the patient had been on a stable dose for at least 4 weeks prior to randomization.
Outcomes: The primary outcome was mPDAI remission at week 14, defined as total mPDAI score of ≤ 4 with a reduction of at least 2 points from baseline.
Secondary endpoints included, but not limited to: (a) mPDAI remission at week 34; (b) PDAI remission at weeks 14 and 34 (PDAI ≤ 6 with a reduction of at least 3 points from baseline); (c) time to PDAI remission; (d) partial mPDAI response (reduction from baseline mPDAI total score of at least 2 points at weeks 14 and 34); and (e) mean changes in the total mPDAI score, endoscopic subscore, and histologic subscore at weeks 14 and 34.
Data Analysis: Modified intention-to-treat analysis, including patients who were randomized and received at least 1 dose of vedolizumab or placebo, was performed. For the primary endpoint, the incidence of mPDAI remission at week 14 was compared using a Fisher’s exact test. Stratified analyses for the primary endpoint according to additional antibiotic use were also performed with a Cochran-Mantel-Haenszel chi-square test. For other dichotomous variables, unadjusted and adjusted between-group differences were calculated using a Cochran-Mantel-Haenszel test with corresponding 95% confidence intervals.
Funding: Takeda, the manufacturer of vedolizumab, had a role in study design and funded study investigators.
Results: Between October 2016 and March 2020, 165 patients were assessed for eligibility. Of these, 102 patients were enrolled, underwent randomization (51 vedolizumab group, 51 placebo group), and received at least 1 dose of vedolizumab or placebo. Eight patients in each group discontinued treatment due to lack of efficacy. Baseline characteristics were similar in both arms: with 84% White, median age 42-45 years of age, 69% male, mean mPDAI score 8.1. At baseline, 10% of patients in the vedolizumab group and 16% of patients in the placebo group were receiving stable doses of steroids. Overall, 59% of patients in the vedolizumab group and 37% of patients in the placebo group received additional antibiotics during weeks 15-34 of the study in addition to the universally prescribed ciprofloxacin from weeks 0-4.
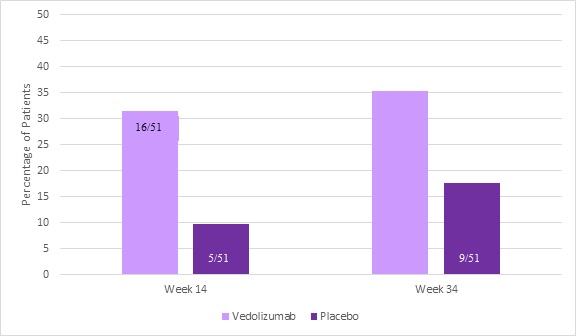
At week 14, a significantly higher proportion of patients in the vedolizumab group achieved mPDAI remission as compared to those in the placebo group: 31% vs 10%, P=0.01 (Figure 1). The percentage of patients who achieved mPDAI remission at week 34 (35% vs 18%, respectively), PDAI remission at weeks 14 and 34, and mPDAI response at weeks 14 and 34 favored vedolizumab.
As compared to the placebo group, the incidence of upper respiratory infection (10% vs 2%) and headache (20% vs 6%) was numerically higher in the vedolizumab group.
Note: *mPDAI includes clinical assessment of stool frequency, rectal bleeding, fecal urgency or abdominal cramping, fever; and endoscopic assessment for edema, granularity, friability, loss of vascular pattern, mucosal exudate, and ulcerations in the pouch body. It excludes 1 domain that is part of the original PDAI: histologic assessment of severity of polymorphonuclear inflammatory infiltrate and number of ulcers per lower power field.
COMMENTARY
Why Is This Important?
Chronic pouchitis is a common complication of IPAA for UC that greatly impairs quality of life. Acute pouchitis, characterized by fecal urgency, diarrhea, and/or abdominal-pelvic discomfort, occurs in more than 50% of patients within 5 years, and approximately 20% develop chronic symptoms that last longer than 4 weeks. The mainstay of therapy is recurrent or chronic use of antibiotics, usually ciprofloxacin or amoxicillin-clavulanic acid, for 14- to 28-day courses. Antibiotic-resistant patients may be treated with oral corticosteroids, specifically budesonide, or topical corticosteroids, although these patients are frequently hesitant to use enemas or suppositories. While uncontrolled studies suggest that biologic therapies are safe and effective for long-term management of chronic pouchitis1, there are no FDA-approved therapies for chronic pouchitis. This is the first RCT demonstrating the safety and efficacy of a biologic therapy for treatment of chronic pouchitis.
Key Study Findings
This effect appeared sustained over the 34-week trial. While vedolizumab appeared overall safe, a higher rate of headaches and upper respiratory infections were reported in the vedolizumab group vs placebo.
Caution
While all patients in the trial received 4 weeks of ciprofloxacin with no antibiotics allowed during weeks 5-14, additional antibiotic use was allowed during weeks 15-34. The trial reported higher rates of additional antibiotic use in the vedolizumab group vs the placebo group (59% vs 37%, respectively). As antibiotics are the current mainstay of therapy for chronic pouchitis, their use may have confounded the results of this trial. The authors used a stratified analysis by additional antibiotic use to try to account for this, and did not find a substantial difference between groups, although the number of patients in each group was small.
My Practice
The EARNEST trial is an important step in the treatment algorithm of chronic pouchitis after IPAA for UC and substantiates uncontrolled data that vedolizumab is a safe and effective therapy for antibiotic-refractory or -dependent disease. However, my enthusiasm for this trial is tempered by the rate of additional antibiotic use in the vedolizumab group. I consider vedolizumab the initial biologic agent for chronic pouchitis after IPAA for UC in antibiotic-refractory or -dependent patients.
Clinicians should do pouchoscopy and ensure that no clear stricturing or penetrating features of Crohn’s disease of the pouch is present since occasional patients may be diagnosed with Crohn’s after their IPAA. If stenoses are present in the pouch inlet, mid-pouch body, or neo-terminal ileum, I would treat the patient for stricturing small bowel Crohn’s disease and consider first-line treatment with an anti-TNF or anti-IL12/23 or IL-23 depending on co-morbidities and prior biologic exposure.
For Future Research
While the EARNEST trial supports that vedolizumab is superior to placebo for the management of chronic pouchitis over 34 weeks, head-to-head trials of biologic therapies are required to better define the management algorithm for this debilitating disease. Furthermore, long-term trial data is required to understand the role of biologic therapy in long-term outcomes including patient quality of life, and need for repeat surgery such as end-ileostomy or pouch revision.
Conflicts of Interest
Dr. Burke is a consultant for OM1 and has participated in an advisory board for Bristol Myers Squibb.
Dr. Kochar is an advisory board member for Pfizer Pharmaceuticals.
@silvio_silvio75 (Silvio Danese)
@DiscoverIBD (Brian Bressler)
REFERENCE
- Shehab M, Alrashed F, Charabaty A, Bessissow T. Biologic therapies for the treatment of post-ileal pouch anal anastomosis surgery chronic inflammatory disorders: Systematic review and meta-analysis. J Can Assoc Gastroenterol 2022;5(6):287-296.

