Upadacitinib Is Effective for the Induction and Maintenance of Moderate-to-Severe Crohn’s Disease
 Rahul S. Dalal, MD, MPH1 and Jessica R. Allegretti, MD, MPH, FACG2
Rahul S. Dalal, MD, MPH1 and Jessica R. Allegretti, MD, MPH, FACG2
1Division of Gastroenterology, Hepatology and Endoscopy, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
2 Medical Director, Crohn’s and Colitis Center, Division of Gastroenterology, Hepatology and Endoscopy, Department of Medicine, Brigham and Women’s Hospital; Associate Professor of Medicine, Harvard Medical School, Boston, MA
This summary reviews Loftus EV Jr, Panés J, Lacerda AP, Peyrin-Biroulet L, et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med 2023 May 25;388(21):1966-1980.
Correspondence to Jessica Allegretti, MD, MPH. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Is upadacitinib effective for the induction and maintenance of moderate-to-severe Crohn’s disease?
Design: Three clinical trials were included: U-EXCEL, U-EXCEED, and U-ENDURE. U-EXCEL and U-EXCEED were both 12-week, double-blinded placebo-controlled, induction of remission trials with 12-week extended treatment periods for those without initial clinical response. In U-EXCEED, all patients had prior failure of biologic therapies, while in U-EXCEL, patients had to have failed either biologic (approximately 45%) or conventional therapies (approximately 55%). U-EXCEED included an additional 12-week open-label single-group induction period. Patients with clinical response or clinical remission after 12 weeks of upadacitinib induction were enrolled in U-ENDURE, which was a 52-week double-blinded, placebo-controlled maintenance of remission trial.
Setting: Two hundred seventy-seven sites across 43 countries.
Patients: Adults age 18-75 years with a diagnosis of moderate-to-severe Crohn’s disease for at least 3 months.
Interventions: Upadacitinib (45 mg once daily for U-EXCEL and U-EXCEED; 15 mg once daily or 30 mg once daily for U-ENDURE) vs placebo.
Outcomes: The primary outcomes were clinical remission (Crohn’s Disease Activity Index [CDAI] < 150) and endoscopic response (decrease in Simple Endoscopic Score for Crohn’s Disease [SES-CD] of >50% from baseline) at week 12 of induction and week 52 of maintenance. Secondary outcomes included, but were not limited to: clinical response (decrease of >100 point in CDAI from baseline), steroid-free CDAI clinical remission, endoscopic remission, resolution of extraintestinal manifestations, deep remission (both clinical remission and endoscopic remission), and maintenance of CDAI clinical remission.
Data Analysis: U-EXCEL and U-EXCEED analyses were performed using modified intention-to-treat populations, which included all patients who were randomized and received at least one dose of upadacitinib or placebo. Analyses for U-ENDURE were performed for those who completed the week 52 visit. Categorical data were analyzed using Cochran-Mantel-Haenszel models and continuous data were analyzed using mixed-effects repeated-measures models.
Funding: The study was funded by AbbVie, manufacturer of upadacitinib.
Results: In total, 526 patients were randomized in U-EXCEL, 495 in U-EXCEED, and 502 in U-ENDURE. At week 12, significantly higher proportions of patients who received 45 mg upadacitinib achieved clinical remission (50% vs 29% in U-EXCEL, 39% vs 21% in U-EXCEED) and endoscopic response (45% vs 13% in U-EXCEL, 35% vs 4% in U-EXCEED) compared to placebo. At week 52 (U-ENDURE only), higher proportions of patients achieved clinical remission and endoscopic response with 15 mg upadacitinib or 30 mg upadacitinib compared to placebo (37% and 48% vs 15% for clinical remission; 28% and 40% vs 7% for endoscopic response). In U-ENDURE RCT, the 30mg upadacitinib arm also demonstrated a significant reduction in extra-intestinal manifestations of Crohn’s disease. Herpes zoster infections were more common in the 45 mg and 30 mg upadacitinib groups compared to placebo. There was no evidence of cardiovascular or thromboembolic complications with upadacitinib. A summary of key efficacy outcomes is presented in Figure 1.
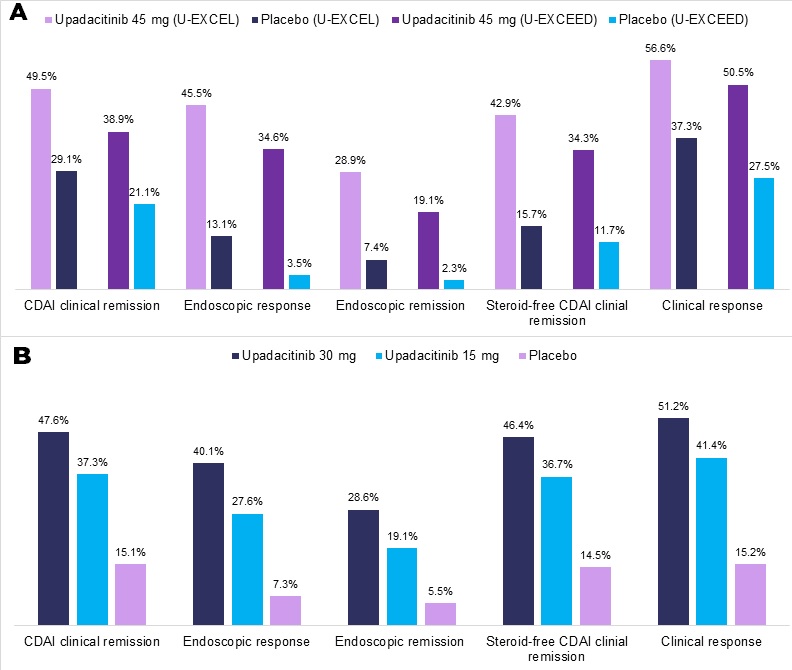
Figure 1. (A) Selected 12-week induction outcomes (U-EXCEL and U-EXCEED). (B) 52-week maintenance outcomes (U-ENDURE) of upadacitinib vs placebo for Crohn’s disease.
CDAI, Crohn’s Disease Activity Index.
COMMENTARY
Why Is This Important?
The available number of advanced therapies for inflammatory bowel diseases has rapidly expanded over the last decade to include biologics as well as small molecule agents. Prior to upadacitinib, only biologics, which are susceptible to immunogenicity and subsequent loss of efficacy, have been available for the treatment of moderate-to-severe Crohn’s disease.1 Tofacitinib, a Janus kinase inhibitor approved for the treatment of ulcerative colitis, did not demonstrate efficacy for Crohn’s disease compared to placebo.2 Upadacitinib represents the first Janus kinase inhibitor and small molecule agent that has received FDA-approval for Crohn’s disease. The novel mechanism of action presents an opportunity to successfully treat cases of Crohn’s disease that have been refractory to anti-tumor necrosis factor (TNF), anti-integrin, and/or anti-interleukin agents, but may also be used immediately after anti-TNF failure. It is also the only orally administered maintenance treatment available for this population that can be stopped and started without the risk of anti-drug antibody formation.
Key Study Findings
Infections such as herpes zoster occurred more commonly in upadacitinib treatment groups compared to placebo.
Caution
While the safety outcomes of upadacitinib appear to be similar to the known safety profile of Janus kinase inhibitors, the limited sample sizes and follow-up period cannot exclude a significantly higher risk of rare or delayed adverse effects, such as cancer. Additionally, based on the data presented in these trials, it is not clear how effective upadacitinib is for certain Crohn’s disease phenotypes, such as fistulizing disease.
My Practice
Due to our own observations regarding the effectiveness of upadacitinib in the treatment of moderate-to-severe ulcerative colitis, including those refractory to other advanced therapies, we are inclined to utilize upadacitinib in cases of luminal colonic Crohn’s disease after anti-TNF failure. However, in certain populations, such as the elderly and those at higher risk for infection or malignancy, we may first consider biologics with more established and favorable safety profiles (i.e., vedolizumab, ustekinumab, and risankizumab).
For Future Research
Future research should examine the efficacy and safety of upadacitinib for challenging Crohn’s disease phenotypes, such as perianal fistulizing disease, as well as in pregnancy. Head-to-head clinical trials and real-world comparative effectiveness studies are also needed to help determine the optimal positioning of upadacitinib relative to other advanced therapies for Crohn’s disease.
Conflict of Interests
Dr. Dalal has received grant support from Janssen Pharmaceuticals and Pfizer Pharmaceuticals and has served as a consultant for Centaur Labs.
Dr. Allegretti has received grant support from Janssen Pharmaceuticals, Pfizer Pharmaceuticals, and Merck Pharmaceuticals, and has served as a consultant for Janssen Pharmaceuticals, Pfizer Pharmaceuticals, AbbVie Pharmaceuticals, Ferring Pharmaceuticals, Merck Pharmaceuticals, Bristol Myers Squibb, Seres Therapeutics, Finch Therapeutics, Iterative Scopes, and Takeda Pharmaceuticals.
@JeanFredericCo1
@RPanaccione
REFERENCES
- Barberio B, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in induction and maintenance of remission in luminal Crohn’s disease: systematic review and network meta-analysis. Gut 2023;72(2):264-274.
- Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 2017;66(6):1049-1059.

