In Case You Missed It
Budesonide Oral Suspension Improves Outcomes in Eosinophilic Esophagitis
 Swathi Eluri, MD, MSCR
Swathi Eluri, MD, MSCR
Senior Associate Consultant, Mayo Clinic Florida, Jacksonville, FL; and Adjunct Assistant Professor of Medicine, University of North Carolina School of Medicine, Chapel Hill, NC
This summary reviews Hirano I, Collins MH, Katzka DA, et al. Budesonide oral suspension improves outcomes in patients with eosinophilic esophagitis: results from a phase 3 trial. Clin Gastroenterol Hepatol 2022;20(3):525-534.e10
Access the article through PubMed
Correspondence to Swathi Eluri, MD, MSCR, Associate Editor. Email: EBGI@gi.org
Keywords: eosinophilic esophagitis, topical corticosteroid, budesonide
STRUCTURED ABSTRACT
Question: What is the efficacy and safety of budesonide oral suspension (BOS) 2.0 mg twice daily compared with placebo in adolescents and adults with eosinophilic esophagitis (EoE) over a 12-week period?
Design: This is a phase 3, multicenter, randomized, double-blind, placebo-controlled trial conducted between 2015 and 2019. Eligible patients were randomized in a 2:1 manner to receive BOS 2.0 mg twice daily (10 mL at a concentration of 0.2 mg/mL) or placebo for 12 weeks.
Setting: Sixty-six centers in the United States.
Patients: Patients were 11–55 years of age with histologic evidence of EoE, defined as having ≥15 eosinophils/high-power field [eos/hpf] from at least 2 levels of the esophagus during screening. To be included, patients also need to have dysphagia on at least 4 days in any 2 consecutive weeks during screening and in the 2 weeks before randomization measured using the Dysphagia Symptom Questionnaire (DSQ).
Intervention: BOS 2.0 mg twice daily vs placebo for 12 weeks. BOS is an immediate release topical steroid, and the viscous formulation allows for longer contact time of the drug to the esophageal mucosa and thereby optimizing delivery.
Outcomes: Co-Primary Efficacy Endpoints were: (a) proportion of stringent histologic responders, defined as <6 eos/hpf across all available esophageal levels (proximal, middle, or distal); and (b) proportion of patients experiencing a significant improvement in dysphagia symptoms, defined as >30% reduction in their DSQ score from baseline.
Key secondary efficacy endpoint was change in DSQ Score from baseline to week 12 of treatment, providing insight into the overall improvement in dysphagia symptoms over the study period. Additional secondary efficacy endpoints included proportion of full responders, defined as patients achieving both a stringent histologic response (≤6 eos/hpf) and a dysphagia symptom response (≥30% reduction in DSQ score), mean change in EoE Endoscopic Reference Score (EREFS), proportion of patients achieving deep histologic response (≤1 eos/hpf) or histologic response (<15 eos/hpf), and mean change in EoE Histology Scoring System (EoEHSS) Total Score Ratios from baseline to week 12 of therapy.
In addition to monitoring adverse events, including esophageal and oral candidiasis, at every study visit, safety assessments included dual x-ray absorptiometry for bone mineral density (for patients 11–17 years of age), and routine clinical laboratory and adrenocorticotropic hormone (ACTH) stimulation tests.
Data Analysis: Modified intention-to-treat analysis and per-protocol analysis were performed. Co-primary efficacy endpoints were compared using the Cochran–Mantel–Haenszel (CMH) test, stratified for several factors including age and dietary therapy. An analysis of covariance model was generated for the key secondary efficacy endpoints, with treatment and age group as factors and the baseline DSQ score as a continuous covariate.
Funding: Shire ViroPharma, Inc., a member of the Takeda group of companies, manufacturer of budesonide oral suspension.
Results: Three hundred and eighteen patients (BOS, n = 213; placebo, n = 105) were randomized and received ≥1 dose of study treatment. Mean age was 34 years old with 13% <18 years old; 60% male; mean peak eosinophil count was 75 eos/hpf; 10% currently on diet restriction and 84% were concurrently using proton pump inhibitors (PPIs).
Patients treated with BOS were more likely to be responders vs placebo-treated patients for both co-primary endpoints. For strict histologic response, responder rate was 53.5% vs 1.0%, respectively; Δ53% [95% confidence interval (CI), 43.8%–59.5%]; P <.001. For >30% reduction in dysphagia symptom questionnaire score, responder rate was 52.6% vs 39.1%, respectively; Δ13% [95% CI, 1.6%–24.3%]; P =.024. (Figure 1). Results were similar for the per-protocol set. Full response, defined as achieving both stringent histologic response and >30% reduction in dysphagia symptom questionnaire score, occurred more frequently with budesonide oral suspension: 30% vs 0%, respectively, P <0.001.
BOS-treated patients also had greater improvements in least-squares mean DSQ scores and EREFS over 12 weeks than placebo-treated patients: DSQ, –13.0 (SEM 1.2) vs –9.1 (SEM 1.5) (Δ–3.9 [95% CI, –7.1 to –0.8]; P =.015); EREFS, –4.0 (SEM 0.3) vs –2.2 (SEM 0.4) (Δ–1.8 [95% CI, –2.6 to –1.1]; P <.001).
BOS was well tolerated with mild to moderate treatment-emergent adverse events, which were comparable in BOS (61%) and placebo (61%) groups after 12 weeks.
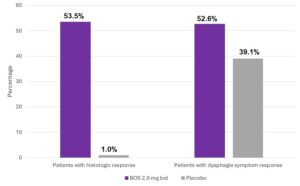
Figure 1. Co-primary endpoints.
COMMENTARY
Why Is This Important?
EoE is a chronic, immune-mediated, inflammatory disease of the esophagus that can lead to esophageal dysfunction including symptoms of dysphagia, esophageal strictures, and food impactions.1 Management typically involves PPIs and elimination diets.2 Off-label use of topical corticosteroids from inhalers, originally formulated for asthma, is also common. However, these inhaled formulations aren’t optimized for esophageal delivery by swallowing an inhaled dose. This potentially leads to inadequate treatment response and associated risks of uncontrolled disease activity such as food impaction and reduced responsiveness to dilation. Although some compounding pharmacies will create an oral suspension, obtaining insurance coverage for off-label medications can be challenging to obtain.
Therefore, Food and Drug Administration (FDA)-approval of a budesonide oral suspension, which was partly based on this study, addresses a significant unmet medical need for more effective EoE treatments. It is the first US phase 3 trial of a corticosteroid therapy for EoE and the largest clinical trial for EoE at time of publication.
Key Study Findings
Key secondary efficacy endpoints, such as deep histologic response (≤1 eos/hpf), histologic response (≤15 eos/hpf), reduction in EREFS score, and maximum peak eosinophil count, also favored BOS over placebo. Additionally, BOS-treated patients showed greater reductions EoEHSS scores compared to placebo.
Caution
The study population is heterogeneous in terms of being on prior or concomitant medical or dietary therapies for EoE. The group also comprised of those with more severe disease so there might be a component of selection bias as it is unclear if the results can be generalizable to those with milder disease forms of EoE. Additionally, it is important to recognize that patients were only followed for the predetermined endpoint of 12 weeks, so we do not have longer term data regarding side effects and possible complications with maintenance therapy for BOS.
My Practice
Topical steroids are recommended as one of the treatment options in the management of EoE per societal guidelines. However, there have been limitations with the lack of availability of budesonide oral suspension other than in select specialty pharmacies which are not accessible to everyone. Alternate corticosteroid formulations designed for used in other conditions, such as asthma, typically are unsuccessful in achieving optimal esophageal mucosal delivery, which can affect treatment outcomes. Having BOS approved by the FDA based on the results of this study leads to easier access for EoE patients for a viable steroid therapy.
In most cases, I will initiate high dose PPI therapy for patients with EoE as the first step with a repeat endoscopic exam with biopsies after 8-12 weeks of therapy. This is important because improvement in dysphagia symptoms may not correlate with histologic remission. Achieving histologic remission is believed to be important to minimize the development of esophageal strictures that could require dilation. I include dietary therapy and/or topical corticosteroids as second line treatment depending on patient choice. When using elimination diets, I’ll frequently start with elimination of dairy and then may also eliminate wheat products before having patients start a more restrictive 6-food elimination diets. I’ll usually have these patients work with a dietitian to improve compliance. Finally, I usually reserve dupilumab, an FDA-approved monoclonal antibody injected subcutaneously weekly, for more severe and refractory cases of EoE.
For Future Research
Future studies with long term follow-up data could help assess the sustained efficacy and long-term safety outcomes of BOS in management of EoE. Additional assessments in the pediatric population only can help provide insights regarding dosing and side effects. Comparative effectiveness studies evaluating BOS to other treatments for EoE such as PPIs and dupilumab can help identify the most appropriate treatment option for differing patient population or phenotypes of EoE. Finally, the new American College of Gastroenterology guidelines on the management of EoE are forthcoming and may further direct optimal management.
Conflict of Interest
None to report
REFERENCES
- Dellon E.S. Liacouras C.A. Molina-Infante J. et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology 2018;155:1022-1033
- Hirano I. Chan E.S. Rank M.A. et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology 2020;158:1776-1786.

