Inching Closer to a NASH Cure: Daily Semaglutide Achieves Resolution of NASH but Not Fibrosis after 72 Weeks

Sonali Paul, MD, MS
Associate Professor of Medicine, Division of Gastroenterology, Hepatology & Nutrition, University of Chicago Medicine, Center for Liver Disease, Chicago, Illinois
This article reviews Newsome PN, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide. N Engl J Med 2021;384(12):1113-24. https://pubmed.ncbi.nlm.nih.gov/33185364/
Correspondence to Sonali Paul, MD, MS, Associate Editor. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Is semaglutide, a glucagon-like-peptide-1 (GLP-1) receptor agonist currently used for the treatment of type II diabetes mellites (DM) and weight loss,1 effective treatment in patients with biopsy-proven nonalcoholic steatohepatitis (NASH) and fibrosis?
Design: This was a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial for 72 weeks that included patients with biopsy confirmed NASH and hepatic fibrosis (stage F1, F2, or F3 but not F4/cirrhosis). Patients were randomized in a 3:3:3:1:1:1 ratio to receive varying doses of semaglutide or placebo.
Setting: This trial was conducted across 16 countries at 143 sites.
Patients: There were 320 patients 18 to 75 years old (mean 55 years) with biopsy confirmed NASH and fibrosis (28% with F1, 22% with F2, and 49% with F3), with or without DM (glycosylated hemoglobin, HgA1c, <10%), and a body mass index (BMI) of >25. The majority of patients were women (61%), White (78%), and had DM (62%) with a mean BMI of 36. Patients with other chronic liver disease, excessive alcohol consumption, and on other modifying treatments (such as Vitamin E or pioglitazone) were excluded from the trial.
Interventions/Exposure: Patients received daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg or placebo. All subjects received routine nutrition and physical activity counseling.
Outcome: The primary end point was NASH resolution without fibrosis worsening; secondary endpoint was fibrosis improvement (of at least 1stage) without NASH worsening.
Data Analysis: Intention-to-treat and per-protocol analysis reported. Only patients with F2 or F3 fibrosis at baseline were analyzed for the primary endpoint of NASH resolution (to more closely match the intended target population as determined by the US Food and Drug Administration and European Medicines Agency).
Funding: Novo Nordisk, who manufactures semaglutide, was involved in trial design, site monitoring, data collection, and analysis.
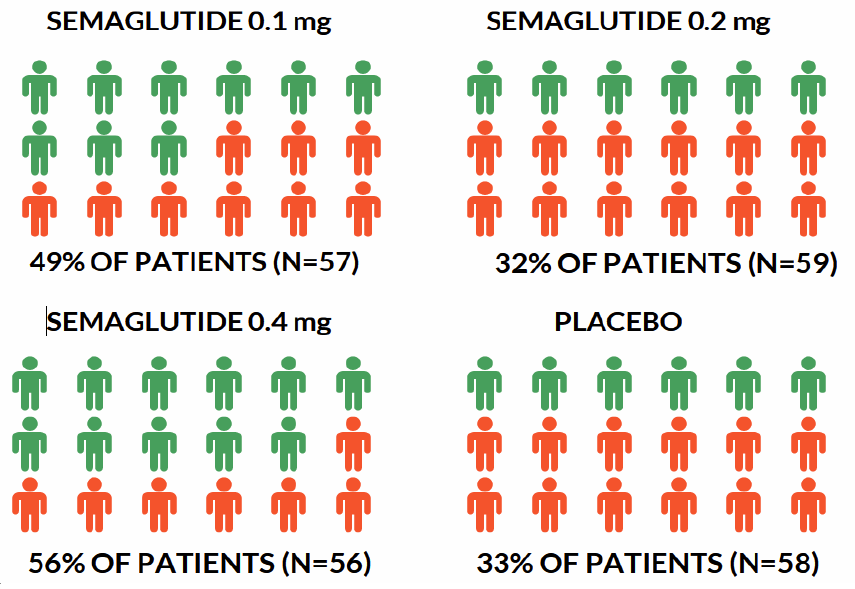
Results: Significantly more patients in the semaglutide groups then in the placebo groups achieved NASH resolution without worsening of F2 or F3 fibrosis with the greatest results seen in the semaglutide 0.4mg group (59% vs 17% in placebo; Figure 1). The study did not achieve their secondary endpoint; no semaglutide groups had significantly greater improvement in fibrosis without worsening NASH compared to placebo (Figure 2). Patients in the semaglutide groups also had dose dependent reductions in HgA1c, liver tests, hepatic stiffness values (based on transient elastography), and body weight (13% in the semaglutide 0.4mg group vs 1% in placebo) at 72 weeks.
Figure 1. Resolution of NASH with No Worsening of Liver Fibrosis
Figure 2. Resolution of NASH with No Worsening of NASH
Commentary
Why is this important?
Approximately 30% of the US population has nonalcoholic fatty liver disease (NAFLD) with 83 million people affected and of those, 3.3 million at risk for cirrhosis and its complications.2 While weight loss can reverse NAFLD and NASH, it is difficult to sustain. Currently, there are no FDA approved medications for the treatment of NASH although there are several currently in the pipeline.3
Key Study Findings
More patients on semaglutide, especially higher dose 0.4mg daily, had NASH resolution compared to placebo at 72 weeks. However, semaglutide did not improve hepatic fibrosis in patients with NASH..
Caution
Semaglutide was associated with more GI complications, including nausea (42% vs 11%), constipation (22% vs 12%), and vomiting (15% vs 2%). Cholelithiasis was also seen in about 6% of the semaglutide group (presumably related to weight loss). The placebo rate of fibrosis regression was also very high at 33% for unclear reasons but not uncommonly seen in NASH trials.4 Additionally, semaglutide is often weekly (either in 1mg or 2.4 mg injections) and not daily as designated in this trial.
My practice
In my hepatology practice, I use a multidisciplinary approach in the management of NAFLD. Other causes of fatty liver (including alcohol, hepatitis C, medications, and Wilson’s disease if age appropriate) are ruled out and metabolic risk is stratified with HgA1c and lipid panel, and patients receive transient elastography to stage their fibrosis (if any). Our dieticians also perform an extensive dietary and physical activity inventory. We incorporate the Mediterranean diet (modified to include not more then 30 grams of carbohydrates/meal), 3 cups of drip coffee/day, and 4-5 tablespoons of olive oil / day in addition to physical activity (10,000 steps / day up to 150 minutes of moderate exercise / week). Semaglutide is used as an adjunct to diet and lifestyle interventions for purposes of either weight loss (marketed as Wegovy®; in patients with BMI >30 or >27 with one metabolic co-morbidity) or for diabetes control (Ozempic®, often with the partnership of our endocrinologist) but not specifically used for NASH given the limited data and insurance restrictions. In addition, I always counsel my patients on the association with medullary thyroid cancer and multiple endocrine neoplasm syndrome 2 (MEN2) with the use of GLP-1 agonists; anyone with a personal or family history of such cancers should not use semaglutide. Vitamin E 800 IU/day in patients with biopsy proven NASH (with or without diabetes or cirrhosis) can also be used.5 Other weight loss medications or referral to bariatric surgery are also commonly used in my practice for NAFLD management.
For future research
Weekly semaglutide 2.4mg (the dose for obesity management)1 is currently being investigated for NASH treatment (NCT04822181). Any medication used for the treatment of NASH will need to achieve both NASH and fibrosis resolution, address the co-morbidities associated with NAFLD including metabolic syndrome and cardiovascular disease, and have a tolerable metabolic side effect.3 Semaglutide is close to achieving many of these endpoints.
REFERENCES
1. Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021 Mar 18;384(11):989. https://doi.org/10.1056/NEJMoa2032183.
2. Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. https://doi.org/10.1002/hep.28431.
3. Alkhouri N, Tincopa M, Loomba R, Harrison SA. What Does the Future Hold for Patients With Nonalcoholic Steatohepatitis: Diagnostic Strategies and Treatment Options in 2021 and Beyond? Hepatol Commun 2021; 5(11):1810-23. https://doi.org/10.1002/hep4.1814.
4. Noureddin N, Han MAT, Alkhouri N, et al. Accounting for the Placebo Effect and Optimizing Outcomes in Clinical Trials of Nonalcoholic Steatohepatitis (NASH). Curr Hepatology Rep 2020;19: 63–69. https://doi.org/10.1007/s11901-020-00505-1.
5. Vilar‐Gomez E, Vuppalanchi R, Gawrieh S, et al. ..Vitamin E Improves Transplant‐Free Survival and Hepatic Decompensation Among Patients With Nonalcoholic Steatohepatitis and Advanced Fibrosis. Hepatology 2020; 71: 495–509. https://doi.org/10.1002/hep.30368.