Upadacitinib, a Selective JAK1 Inhibitor, for Moderate-Severe Ulcerative Colitis: Adjusting the Top-Down Treatment Algorithm for Ulcerative Colitis

Jami Kinnucan, MD1 and Philip Schoenfeld, MD, MSEd, MSc (Epi)2
1Senior Associate Consultant, Mayo Clinic, Jacksonville, FL
2Chief (Emeritus), Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI
This summary reviews: Danese S, Vermeire S, Zhou W, et al. Upadacitinib as Induction and Maintenance Therapy for Moderately to Severely Active Ulcerative Colitis: Results from Three Phase 3, Multicentre, Double-Blind, Randomised Trials. Lancet 2022; 399: 2113-28. doi: 10.1016/S0140-6736(22)00581-5
Correspondence to Philip Schoenfeld, MD, MSEd, MSc (Epi), Editor-in-Cheif. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Is upadacitinib (Rinvoq), a selective JAK1 inhibitor, superior to placebo for induction and maintenance of remission in moderately to severely active ulcerative colitis (UC)?
Design: To assess induction of remission at 8 weeks, 2 multicenter, double-blind, placebo-controlled randomized controlled trials (RCTs; U-ACHIEVE substudy 2 and U-ACCOMPLISH) were conducted, and a single multi-center, double-blind, placebo controlled RCT (U-ACHIEVE substudy 3) was performed to assess maintenance of remission at 52 weeks. Randomization stratified for multiple factors, including history of biologic failure, baseline corticosteroid use, and baseline Adapted Mayo Score (<7 vs >7).
Setting: Each RCT was conducted in approximately 200 centers in 35-40 countries across Europe, North and South America, Australasia, Africa and the Asia-Pacific region.
Patients: In the induction of remission RCTs, patients were: (a) 18-75 years old; (b) confirmed UC diagnosis > 90 days; (c) moderate-severe UC based on Adapted Mayo Score of 5-9 with endoscopic subscore of 2-3; and (d) previous inadequate response/loss of response/intolerance to standard UC treatment with 5-ASA, steroid, immunosuppressant, or biologic therapy. Exclusion criteria included active infection, toxic megacolon or prior exposure to JAK inhibitors. Patients who achieved clinical remission after 8 weeks of upadacitinib treatment were eligible for enrollment in the maintenance of remission RCT.
Interventions/Exposure: In the 2 induction of remission RCTs, patients were randomized 2:1 to upadacitinib 45 mg po qd vs placebo for 8 weeks. In the maintenance of remission RCT, patients were randomized 1:1:1 to upadacitinib 30 mg po qd, upadacitinib 15 mg po qd, or placebo for 52 weeks.
Outcome: The primary endpoint was clinical remission defined as Adapted Mayo score < 2 with stool frequency score < 1 and not greater than baseline, rectal bleeding score = 0, and endoscopic subscore < 1 without friability*. Multiple secondary endpoints were assessed, including endoscopic remission and clinical response defined as decrease in Adapted Mayo Score of > 2 points and > 30% from baseline with decrease in rectal bleeding score of > 1 point. In addition to standard safety analyses, pre-specified adverse events of interest were serious infection, herpes zoster, malignancy, major adverse cardiac events (MACE), and venous thromboembolisms.
Data Analysis: Modified intention-to-treat analysis defined as patients who were randomized and received at least one dose of study medication was performed for the primary and secondary endpoints in the induction RCTs. Safety analysis performed for any patient who received study medication in both induction and maintenance RCTs.
Funding: AbbVie Pharmaceuticals.
________________________
*Note: the Adapted Mayo Score assesses rectal bleeding score (0-3), stool frequency score (0-3), and centrally-assessed endoscopy subscore (0-3), but excludes the Physician’s Global Assessment used in the full Mayo score. Therefore, the score range is 0-9 with 9 representing most severe UC.
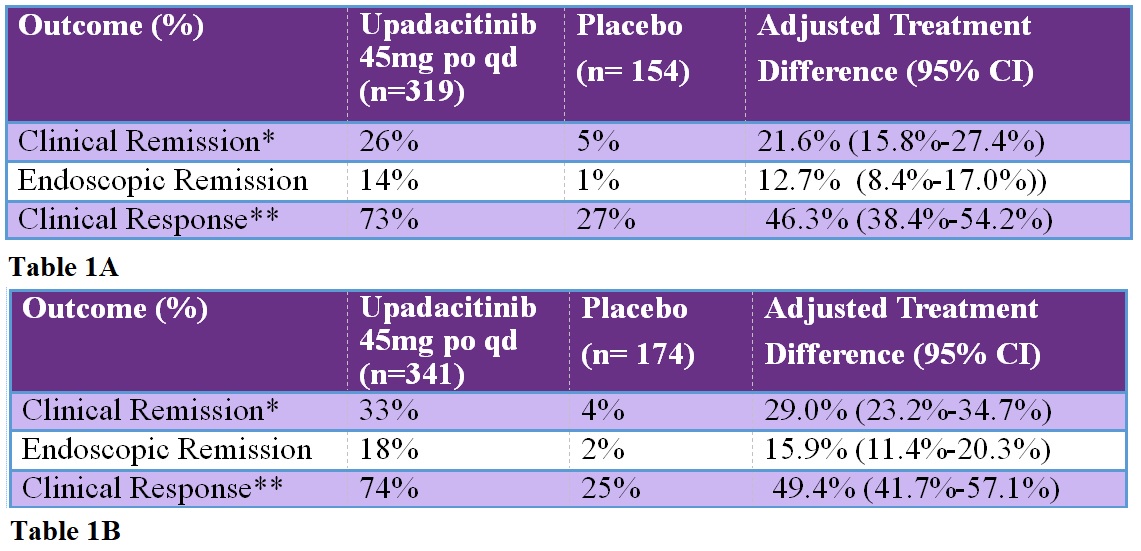
Table: A. Induction of remission at week 8 in U-ACHIEVE substudy 2. B. Induction of remission at week 8 in U-ACCOMPLISH.
*Clinical Remission: Adapted Mayo score < 2 with stool frequency score < 1 and not greater than baseline, rectal bleeding score = 0, and endoscopic subscore < 1 without friability.
**Clinical Response: Decrease in Adapted Mayo Score of > 2 points and > 30% from baseline with decrease in rectal bleeding score of > 1 point.

Figure 1. Maintenance of Remission at Week 52
COMMENTARY
Why Is This Important?
There is an expanding landscape of therapies for UC treatment. Available biological therapies include anti-tumor necrosis factor (TNF) antibody treatments like infliximab (Remicade), adalimumab (Humira), golimumab (Simponi),and anti-integrin antibody treatments like vedolizumab (Entyvio) and anti-interleukin-12/23 antibodies such as ustekinumab (Stelara). Recently, small molecule therapies have also been approved for moderate-severe UC, including sphingosine-1 phosphate inhibitors like ozanimod (Zeposia) and non-selective janus kinase (JAK) inhibitors including tofacinitinib (Xeljanz). Given this expanding menu of therapies, new algorithms are sorely needed to account for the strengths and limitations of these agents and to help gastroenterologists choose the optimal treatment for individual UC patients.
Upadacitinib, a selective JAK1 inhibitor, offers many potential advantages for treating UC.1 First and foremost, it’s quite effective with large absolute increases in clinical remission rates vs placebo after 8 weeks of induction therapy and after 52 weeks of maintenance therapy. Although comparative RCTs are not available, this magnitude of benefit was superior to other biologics and small molecules in 2 recent network meta-analyses.2-3 It’s an oral agent taken once daily, which may be preferable for some patients, and this class of agents has a relatively rapid onset of action.4 As a more selective JAK1 inhibitor, it may minimize toxicities associated with pan-JAK blockade. However, the Food and Drug Administration (FDA) only approved upadacitinib for UC treatment AFTER inadequate response or intolerance to an anti-TNF agent, which is similar to the labelling for tofacitinib for UC, largely due to safety concerns raised in post-marketing safety studies of tofacinitib plus methotrexate in older rheumatoid arthritis patients with cardiovascular risks.
Safety is very important with any new class of drugs, but some context is also important. Safety concerns primarily arose from a planned, post-authorization, safety RCT where tofacitinib was compared to anti-TNF agents in rheumatoid arthritis patients aged > 50 years old with at least one cardiovascular risk factor and on background methotrexate with median follow-up of 4 years. Cancers and MACE were numerically higher with tofacitinib and did not meet non-inferiority criteria.5 Interim analysis also demonstrated an increased risk for venous thromboembolisms in patients with tofacitinib 10 mg bid (vs tofacitinib 5 mg bid or anti-TNF treatment), although overall incidence was low. The incidence of MACE was lower in the tofacitinib UC trials, and no MACE occurred in upadacitinib-treated patients in the induction or maintenance of remission RCTs. Upadacitinib selectively targets JAK1 inhibition and minimizes JAK2 inhibition, which is the kinase whose inhibition is associated with increased platelet count and thrombosis, so the safety of upadacitinib in younger UC patients may differ. Additional safety data from open-label extension trials are forthcoming.
Ultimately, Danese and colleagues are to be congratulated for producing outstanding RCTs as well as completing patient enrollment during the COVID-19 pandemic and getting study patients through a rigorous study protocol. Although the multitude of available UC treatments may create confusion in the treatment algorithm, there is undoubtedly an unmet medical need for many UC patients that will be addressed with upadacitinib.
Clinical remission was significantly more common with upadacitinib 45 mg qd vs placebo in both induction RCTs: 26% vs 5% and 34% vs 4%, respectively, and maintenance of remission was more common with upadacitinib 30 mg and upadacitinib 15 mg vs placebo: 52% and 42% vs 12%, respectively.
Caution
Per FDA prescribing information, upadacitinib is limited to “adults with moderately to severely active ulcerative colitis who have had an inadequate response or intolerance to one or more TNF blockers.” In addition to safety concerns noted above with tofacitinib, a non-selective JAK inhibitor, an increased risk of herpes zoster and cytomegalovirus infection may occur with upadacitinib, and there is inadequate data to determine safety of all small molecule agents during pregnancy.
My Practice
Given the rapid expansion of biologics and small molecule agents to treat moderate to severe UC in the past 5 years, our approach to managing these patients continues to evolve. Our use of upadacitinib is limited to patients who have had an inadequate response or intolerance to at least one anti-TNF therapies. It’s advantageous to have an oral agent with rapid durable response with lack of immunogenicity concerns for these individuals. Therefore, we individualize our approach to patient care by reviewing risks and benefits and conduct shared decision making. If a JAK inhibitor is used, we’ll use either tofacitinib or upadacitinib based on insurance coverage. Anecdotally, we’ve found insurance coverage for upadacitinib quite good recently.
Prior to prescribing upadacitinib, we follow our standard protocol of recommending vaccination against multiple infections, including herpes zoster. In addition to baseline laboratory assessment (CBC, comprehensive metabolic profile), we check lipid parameters and do follow-up lipids at 12 weeks, which is recommended in the FDA prescribing information due to the potential for increases with low-density lipoproteins, high-density lipoprotein, and total cholesterol.
For Future Research
Ongoing RCTs will define efficacy of upadacitinib for Crohn’s disease. Given the increasing number of available agents with different mechanisms of actions, comparative RCTs would be welcome to help establish positioning of therapies as well as longer-term safety data.
Conflict of Interest
Dr. Kinnucan reports serving as a consultant/advisory board member for Janssen Pharmaceuticals, Pfizer Pharmaceuticals, AbbVie Pharmaceuticals, Takeda Pharmaceuticals, and Bristol Myers Squibb Pharmaceuticals. Dr. Schoenfeld reports no conflicts of interest.
REFERENCES
- Ananthakrishnan A. Upadacitinib for Ulcerative Colitis. Lancet 2022; 399: 2077-78.
- Burr NE, Gracie DJ, Black CJ, Ford A. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut 2022; 71: 1976-87.
- Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2022; 7: 161-70.
- Hanauer S, Panaccione R, Danese S, et al. Tofacintinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 139-47.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N Engl J Med 2022; 386: 316-26.
Download the article summary (PDF)

