Tirzepatide For Obesity: “Mounting” Evidence for Substantial Weight Loss

Associate Editor
Sonali Paul, MD, MS
Assistant Professor of Medicine, Division of Gastroenterology, Hepatology & Nutrition, Pritzker School of Medicine, University of Chicago, Chicago, Illinois
This summary reviews: Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide Once Weekly for the Treatment of Obesity. N Engl J Med 2022;387(3):205-216. https://pubmed.ncbi.nlm.nih.gov/35658024/
Correspondence to Sonali Paul, MD, MS, Associate Editor. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Is tirzepatide, a glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist recently approved for Type II diabetes mellitus (DM), safe and effective for weight loss in conjunction with lifestyle interventions in obese individuals without DM?
Design: Phase 3 randomized, double-blind, placebo-controlled trial.
Setting: Nine countries in North and South America, Asia, and Europe.
Patients: Overall, 2,539 adults (mean age 44.9 years) with either body mass index (BMI) > 30 or BMI > 27 and at least 1 weight-related condition (hypertension, obstructive sleep apnea, cardiovascular disease, or dyslipidemia) were included in the study. Participants were approximately 71% White, 67% women, 41% prediabetic, and mean body weight of 104.8 kg and BMI 38.0. Key exclusion criteria included a history of DM and those with a change in body weight of more than 5 kg within 90 days of enrollment.
Exposure/Intervention: Once weekly subcutaneous tirzepatide (5mg, 10 mg, or 15 mg) vs placebo randomized into a 1:1:1:1 ratio plus lifestyle intervention (defined as individual counseling sessions to improve adherence to healthy balanced meals with 500 calorie deficit per day and at least 150 minutes of physical activity per week) for 72 weeks. Initial tirzepatide dose was 2.5 mg once weekly and increased by 2.5 mg every 4 weeks to reach maintenance dosing (20 weeks for 15 mg once weekly dosing).
Outcome: Co-primary endpoints were percentage change in weight from baseline and weight reduction of > 5% or more at week 72.
Data Analysis: Intention-to-treat analysis reported.
Funding: Eli Lilly, manufacturer of tirzepatide, designed and oversaw the study including data collation and analysis.
Results: Mean percentage change in weight was -15.0% (95% confidence interval [CI] -15.9 to -14.2) with 5 mg weekly tirzepatide doses, -19.5% (95% CI -20.4 to -18.5) with 10 mg doses, and -20.9% (95% CI -21.8 to -19.9) with 15 mg doses compared to -3.1% (95% CI -4.3 to -1.9) with placebo at 72 weeks (Figure 1). These differences were statistically significant (P < 0.001) for all doses compared to placebo.
Eighty-five percent of participants (95% CI 82 to 89), 89% (95% CI 86 to 92), and 91% (95% CI 88 to 94) of participants achieved weight reduction of > 5% with 5 mg, 10 mg, and 15 mg of tirzepatide, respectively, compared to 35% (95% CI 30 to 39) with placebo (P < 0.001 for all comparisons compared to placebo). Additionally, 50% (95% CI, 46 to 54) of participants on 10mg group and 57% (95% CI, 53 to 61) in the 15 mg groups had body weight reduction of > 20% compared with only 3% (95% CI 1 to 5) in the placebo group (P <0.001 for all comparisons with placebo).
Importantly, tirzepatide also improved cardiometabolic parameters (including waist circumference, systolic and diastolic blood pressure, fasting insulin, and lipid levels) and mean reduction in total body fat mass (33.9% with tirzepatide compared to 8.2% with placebo). Ninety-five percent of patients with prediabetes had improved glucose levels (compared to 62% in placebo). Gastrointestinal side effects (nausea, vomiting, diarrhea) were more common in the tirzepatide group vs placebo and occurred in the setting of higher doses.
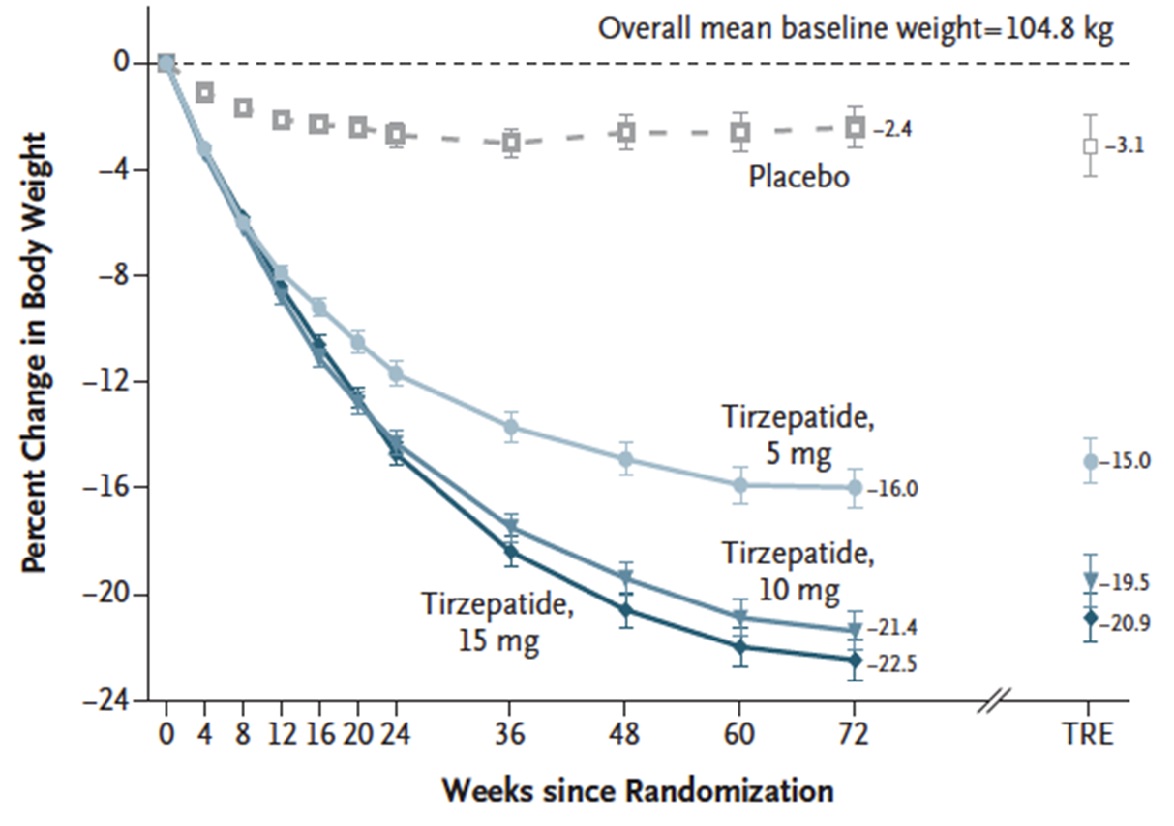
Figure 1. Percent change in body weight
COMMENTARY
Why Is This Important?
Obesity is a global health epidemic resulting in myriad complications including type 2 diabetes, cardiovascular disease, and nonalcoholic fatty liver disease (NAFLD) among many others. Treatments that result in significant weight loss can improve obesity related morbidity and outcomes. These data indicate that tirzepatide is far superior in weight loss as compared to currently available weight loss medications. Older anti-obesity medications currently approved by the FDA include orlistat, phentermine, phentermine-topiramate, naltrexone-bupropion, and liraglutide, and have an average placebo-adjusted weight reduction of 3% to 8.6%.1 A more recently approved GLP-1 receptor agonist, semaglutide 2.4 mg, resulted in a placebo-adjusted weight reduction of 12.4% (and up to 20% in up to a third of patients in that trial).2 Comparatively, tirzepatide—even at the lowest dose of 5 mg—had mean placebo adjusted weight reduction of 11.9% and 36.2% of participants receiving 15 mg dosing achieved weight reduction of 25% or more, comparative to bariatric surgery which results 25 to 30% weight reduction at 1-2 years.3 These data reinforce the importance that this drug will have in the future of obesity management.
Tirzepatide achieved greater mean reduction in body weight of 15% to 21% compared to only 3% in placebo across varying doses at 72 weeks. This is far superior to weight loss observed with any other medication.
Caution
GI adverse events, specifically nausea, diarrhea, and constipation, occurred more frequently with tirzepatide compared to placebo, although only a small percentage of patients discontinued treatment because of adverse events (4.3%, 7.1%, 6.2% of participants receiving 5 mg, 10 mg, and 15 mg tirzepatide doses respectively compared to 2.6% receiving placebo). Additionally, tirzepatide is only currently FDA approved for diabetes and the expectation is it will be approved within the coming year for weight loss. However, insurance coverage and cost remain to be determined. Importantly, any anti-obesity medication should be coupled with counseling from a dietitian on reduced calorie diets and increased physical activity. Finally, the majority of trial participants were White women; further study in other patients’ populations is needed.
My Practice
In my hepatology practice, which includes many NAFLD patients, most obese and overweight patients with 1 additional risk factor are prescribed semaglutide 2.4 mg weekly, which was recently approved by the FDA for obesity. Also, there is some data to also show efficacy in non-alcoholic steatohepatitis (NASH).2 However, insurance often dictates which medications are covered and therefore prescribed. Tirzepatide is approved for patients with diabetes and is being used “off-label” for weight loss in some endocrinology practices.
Until tirzepatide is approved for weight loss and is widely covered from an insurance perspective, bariatric surgery also remains a pillar for the treatment of obesity. Additionally, from a NAFLD perspective, each of these anti-obesity medications is paired with important lifestyle interventions and counseling from a registered dietician.
For Future Research
Better data across all racial/ethnic groups, in men, and in obese diabetic patients are needed. From a GI and hepatology perspective, previous data has shown that tirzeptide can improve NASH-related biomarkers in those with T2DM.4 Tirzepatide is currently being studied in the treatment of NASH in current phase 3 clinical trials (ClinicalTrials.gov Identifier: NCT04166773) and will likely play an role in the future.
Conflicts of Interest
Dr. Paul has no conflicts of interest.
REFERENCES
- American Diabetes Association. Obesity management for the treatment of type 2 diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020; 43: Suppl 1: S89-S97.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med 2021; 384: 989-1002.
- van Rijswijk AS, van Olst N, Schats W, et al. What is weight loss after bariatric surgery expressed in percentage total weight loss (%TWL)? A systematic review. Obes Surg 2021; 31: 3833-47.
- Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 2020;43(6):1352-1355.

