Computer-aided Detection Systems Increase Detection of Non-Advanced Adenomas, but Is It Ready for Prime-time?
 Shria Kumar, MD, MSCE1 and Gottumakkala S. Raju, MD, FACG, FASGE2
Shria Kumar, MD, MSCE1 and Gottumakkala S. Raju, MD, FACG, FASGE2
1Division of Digestive and Liver Diseases, University of Miami Miller School of Medicine, Miami, Florida
2Department of Gastroenterology, Hepatology, and Nutrition, Division of Internal Medicine, MD Anderson Cancer Center, Houston, Texas
This article reviews Repici A, Badalamenti M, Maselli R, et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020;159(2):512-520.e7. PMID: 32371116
Correspondence to Shria Kumar, MD, MSCE, Associate Editor. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Do computer-aided detection systems help detect more adenomas during colonoscopy?
Design: Randomized controlled trial with patients randomized to undergo colonoscopy with or without a computer-aided detection tool, GIGenius™ (Medtronic, Minneapolis, MN) to identify adenomas.
Setting: Three Italian endoscopy centers participating in an organized population-based CRC screening program.
Patients: Outpatients undergoing colonoscopy for screening and surveillance, positive fecal immunohistochemical test, or symptoms prompting colonoscopy.
Interventions: Six experienced endoscopists (>2,000 colonoscopies completed) performed the colonoscopies, and were not blinded to study assignment. GI-Genius™ is a deep learning system that draws endoscopist attention to a potential polyp, by overlaying a “detection box” onto the endoscopy monitor (Figure 1). The endoscopist can then closely examine and resect the lesion as appropriate.
Outcomes: Adenoma detection rate (ADR) was the primary outcome.
Data Analysis: Comparison of ADR between the GI-Genius vs control group, with reported relative risks in an intention-to-treat fashion.
Funding: No funding; Medtronic loaned equipment.
Results: A total of 685 patients underwent randomization, 341 in the GIGenius™ arm and 344 in the control arm. After adjusting for age, gender, and indication, ADR was significantly higher in the GI-Genius™ group, RR, 1.30; 95% CI, 1.14–1.45 (Table 1). This reflects increases in detection of non-advanced adenomas, including lesions < 5mm and 6-9 mm lesions. The GI-Genius was adept in detecting lesions that were polypoid and nonpolypoid, both in the proximal and distal colon. The GI-Genius™ did not detect significantly more advanced adenomas, adenocarcinomas, or sessile serrated lesions, as compared to controls. The authors also evaluated those “polyps” that were resected without any histologic pathology. There was no statistically significant difference in this non-neoplastic resection rate between the 2 groups.

Figure 1.
The GI-Genius™ output appears on the same screen of the endoscopy system and highlights potential adenomas.
Commentary
Why is this important?
Higher ADRs are associated with lower rates of post-colonoscopy colorectal cancer.1,2 Interventions to improve ADR are of intense interest. GIGenius™ is now FDA-approved and can be integrated into existing endoscopy systems. Since it draws endoscopist attention to a potential polyp by overlaying a detection box onto the endoscopy monitor (Figure 1), it may be easily used to improve ADR.3 Multiple artificial intelligence (AI) systems have been described, and international consensus groups have looked to best guide implementation.4,5 Important questions include benchmarks for satisfactory AI and how to standardize thresholds to ensure improved patient outcomes.
Key Study Findings
This is a well-designed randomized controlled trial to evaluate the real-world impact of AI in colonoscopy. GI-Genius significantly improved ADR for non-advanced adenomas (41.6% vs 29.9%, P< 0.001) (Figure 2). However, there was no significant improvement in ADR for advanced adenomas or sessile serrated lesions, although the sample size was too small to adequately assess those endpoints. Importantly, there was no increased detection (or resection) of non-adenomatous polyps with the GI-Genius™.
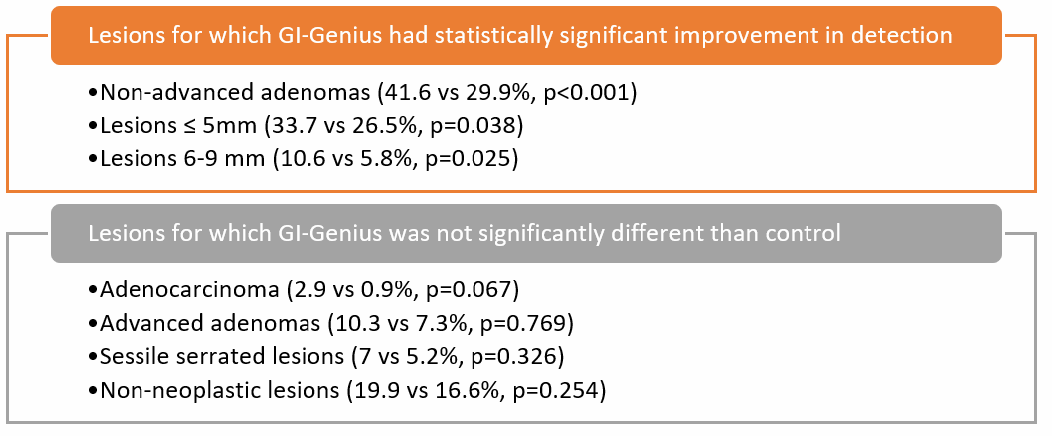
Figure 2.
Comparison of GI-Genius™ vs control, by lesion characteristic.
Caution
The study excluded poorly prepped colons, which underlines the limits of AI in colonoscopy. If the mucosa is not visualized, then AI cannot help. Preparation must be adequate, and the endoscopist needs to flatten folds and assure adequate visualization for the AI tool to work. The endoscopists in this trial are experienced endoscopists, potentially limiting generalizability to wider practice. However, another recent study by Repici, et al demonstrated similar improvements in ADR among less experienced endoscopists (<2,000 colonoscopies).6 In the current study, endoscopists utilized GI-Genius™ on insertion and withdrawal, which is slightly different than the standard practice of inspection on withdrawal alone. Lastly, endoscopists were not blinded, and there could be psychological bias due to this.
My Practice
We have piloted the GI-Genius™ but are not currently using the tool in our practice. Since our group has good ADRs and since the technology is expensive, our centers have not yet opted to incorporate it. This decision may change given the rapid changes in the AI field. If an AI system clearly improves detection of lesions that are harder to detect (e.g., sessile serrated lesions), then we would be even more enthusiastic about the system. There is promise to this end: a recent study by Glissen Brown, et al. evaluating a different AI system demonstrated a decreased miss rate of both adenomas and sessile serrated lesions.7 This also highlights that different AI systems may come to market and comparison between them is important.
For Future Research
The benefit of AI should be evaluated in poor performers with ADR <25% since this group clearly needs interventions for improvement. Standard benchmarks for satisfactory AI in colonoscopy are needed, particularly given the different operating systems, and the cost-effectiveness of implementing AI should be evaluated. The precision of AI is improving rapidly and cost-effectiveness may change when different operating systems are available.
REFERENCES
- Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002;97(6):1296-1308.
- le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut 2014;63(6):957-963.
- Barua I, Vinsard DG, Jodal HC, et al. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy 2021;53(3):277-284.
- Lui TKL, Guo CG, Leung WK. Accuracy of artificial intelligence on histology prediction and detection of colorectal polyps: a systematic review and metaanalysis. Gastrointest Endosc 2020;92(1):11-22 e16.
- Ahmad OF, Mori Y, Misawa M, et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: a modified Delphi method. Endoscopy 2021;53(9):893-901.
- Repici A, Spadaccini M, Antonelli G, et al. Artificial intelligence and colonoscopy experience: lessons from two randomised trials. Gut 2021 Jun 29:gutjnl-2021-324471. doi: 10.1136/gutjnl-2021-324471. Epub ahead of print. PMID: 34187845.
- Glissen Brown JR, Mansour NM, Wang P, et al. Deep learning computer-aided polyp detection reduces adenoma miss rate: A United States Multi-center Randomized Tandem Colonoscopy Study (CADeT-CS Trial). Clin Gastroenterol Hepatol 2021 Sep 14:S1542-3565(21)00973-3.

