Cell-free DNA Blood Test for CRC Screening: A Promising Development Won’t “Eclipse” Current Tools
 Philip Schoenfeld, MD, MSEd, MSc (Epi)
Philip Schoenfeld, MD, MSEd, MSc (Epi)
Chief (Emeritus), Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI.
This summary reviews Chung DC, Gray DM, Singh H, et al. A Cell-free DNA blood-based test for colorectal cancer screening. N Engl J Med 2024; 390: 973-83.
Access the article through PubMed
Correspondence to Philip Schoenfeld, MD, MSEd, MSc. Editor-in-Chief. Email: EBGI@gi.org
Keywords: colorectal cancer, cell-free DNA, screening
STRUCTURED ABSTRACT
Question: What is the sensitivity and specificity of a cell-free DNA (cf DNA) blood test for colorectal cancer (CRC) screening (Shield; Guardant Health, Palo Alto, CA) for detection of Stage I, II, and III CRC and advanced precancerous lesions in average-risk individuals aged 45-84 years old?
Design: Prospective observational diagnostic test study using colonoscopy as the gold standard for detection of CRC and precancerous lesions: ECLIPSE (Evaluation of the ctDNA LUNAR Test in an Average Patient Screening Episode) study.
Setting: Two hundred sixty-five primary care and endoscopy centers in the United States.
Patients: Average-risk individuals aged 45-84 years old scheduled for CRC screening colonoscopy. Key exclusion criteria included: (a) history of inflammatory bowel disease; (b) family history of CRC in first-degree relative; (c) prior history of adenomatous polyps; and (d) currently up-to-date with CRC screening (e.g., had a normal screening colonoscopy < 9 years).
Interventions/Exposure: Whole blood samples (30-80 ml) were collected and shipped at ambient temperatures to central biorepository, processed to plasma, and then stored at -80°C until the assay was performed. The assay evaluates extracellular DNA molecules in the plasma that have been released from tissue into the bloodstream: aberrant DNA methylation status, aberrant DNA fragmentation patterns, and pathogenic variants in Kirsten rat sarcoma virus (KRAS) and adenomatous polyposis coli (APC) genes. Using these data and a logistic regression model, a binary outcome (abnormal signal detected or normal signal detected) is reported.
Outcome: Coprimary outcomes were sensitivity for CRC, including Stage I, II, and III, and specificity for advanced precancerous lesions, defined as adenomas >10 mm, adenoma with villous histology or high-grade dysplasia, carcinoma in situ, or serrated lesion >10 mm. The secondary outcome was sensitivity for advanced precancerous lesions.
Data Analysis: Sensitivity (percentage of individuals with the disease who have a positive test) and specificity (percentage of individuals without the disease who have a negative test) with corresponding 95% confidence intervals (CIs) were calculated with standard formulas. [Note: for previous US Food and Drug Administration (FDA)-approved CRC screening tests, a test was considered acceptable if the lower boundary of the 95% CI for CRC sensitivity was >65% and if the lower boundary of 95% CI for specificity of advanced precancerous lesions was >85%.
Funding: Guardant Health, manufacturer of Shield, cf DNA blood-based test.
Results: Between October 2019 and September 2022, 22,877 patients were enrolled, producing 65 individuals with CRC; 74% (48/65) had stage I, II, or III CRC). An additional 10,193 participants without CRC were randomly selected to complete clinical validation of cf DNA blood-based test. Among this group, 7,861 met all inclusion and exclusion criteria, had complete colonoscopies, and evaluable cf DNA blood-based tests. This final study cohort had mean age of 60 years old (range 45-84), 54% female, 79% White, and 11.4% had a positive cf-DNA blood-based test.
For CRC Stage I-III, 87.5% (42 of 48) had a positive cf-DNA blood-based test. This includes 100% sensitivity for Stage II CRC (14/14) and Stage III CRC (17/17), but only 65% (11/17) for Stage I CRC (Table 1). For advanced precancerous lesions (large adenomas, large sessile serrated polyps, villous adenomas or adenomas with high-grade dysplasia or carcinoma in situ), 13.2% (147 of 1,116) had a positive test. Approximately 10% of participants had a false positive test, defined as positive cf-DNA blood-based test, but no adenomas, advanced precancerous lesions or CRC found on colonoscopy.
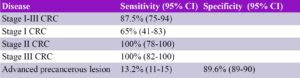
Table 1. Diagnostic test characteristics. CI, confidence interval; CRC, colorectal cancer
COMMENTARY
Why Is This Important?
Only about 59% of the eligible US population is up-to-date with CRC screening, equating to more than 40 million unscreened individuals. Therefore, new interventions to improve screening are sorely needed.1 Given the relative lack of adherence with annual fecal immunochemical tests (FIT) as well as the desire of some patients to avoid colonoscopy with the associated bowel preparation, sedation, and time missed from work, blood-based tests for CRC screening offer the potential for a convenient and easily accessible tool.
I commend the investigators for completing the ECLIPSE study and expanding the science of CRC screening. Currently, the cf-DNA blood-based test can be ordered by physicians, but it’s not covered by Medicare. Given the relatively low sensitivity of cf-DNA blood-based tests for Stage I CRC, poor sensitivity for advanced adenomas, and uncertainty around insurance coverage, this test won’t soon supplant other CRC screening tools. In contrast to this test, the newest version of multi-target stool DNA tests2 demonstrates sensitivity of almost 44% for advanced adenomas with sensitivity for Stage I-III CRC of 92.7% (95% CI 85-97) while also being covered by Medicare and commercial insurers.
For CRC Stage I-III, the sensitivity of the cf-DNA blood-based test was 87.5% (95% CI 75-94), consistent with 42 of 48 individuals with Stage I-III CRC having a positive cf-DNA blood-based test. However, the sensitivity for Stage I CRC was only 65% (95% CI 41-83) since only 11 of 17 individuals with Stage I CRC had a positive test. This is not a useful test for identifying advanced adenomas since the sensitivity is 13.2% (95% CI 11-15) with only 147 of 1,116 individuals having a positive test.
Caution
Although the manufacturer of cf-DNA blood-based tests recommends performance every 3 years, it’s unclear to me how that interval was determined. Also, since approximately 10% of average-risk individuals will have a false-positive test and since other solid-tissue tumors may release abnormal cf-DNA fragments into blood, it’s unclear if any additional cancer screening or diagnostic testing should be performed when a patient has a positive test followed by a normal colonoscopy.
My Practice
The mainstay of CRC screening for gastroenterologists is colonoscopy, a CRC prevention tool. I do see average-risk individuals in clinic who are fearful of colonoscopy, sedation, or simply doing the bowel preparation and want a non-invasive alternative. For these individuals, annual FITs are certainly appropriate cancer detection tools, although the new version of multi-target stool DNA tests are also reasonable, especially since the latest version has superior sensitivity to FIT for CRC and advanced adenomas.
Nevertheless, when explaining the benefits and limitations of different CRC screening tests to patients, the best test is one that the patient completes. If the patient wants a non-invasive test but doesn’t want to obtain a sample from voided stool, then I might offer a blood-based test as long as the patient agreed to get a colonoscopy if the blood-based test was positive and if the patient could pay out-of-pocket for the test, which is not currently covered by Medicare and most commercial insurers.
For Future Research
Although this version of the cf-DNA blood-based test may not be appropriate for widespread use, the development of this technology is a huge advance. Again, the investigators should be congratulated for their efforts and ongoing research in fragmentomics is likely to advance our ability to perform cancer screening with blood-based tests. In the interim, research about how to manage or advise individuals with a false positive test and data to validate a 3-year interval between screening tests is needed.
Conflict of Interest
Dr. Schoenfeld previously served as a speaker for EXACT Sciences.
Samir Gupta@issakaMD
Rachel Issaka
REFERENCES
-
- Lo YMD. Cell-free DNA for colorectal cancer screening. N Engl J Med 2024; 390: 1047-50.
- Imperiale TF, Porter K, Zella J, et al. Next-generation multi- target stool DNA test for colorectal cancer screening. N Engl J Med 2024;390:984-93.

