First FDA-Approved NASH Treatment Produces NASH Resolution and Decreases Fibrosis: Results From the Landmark Phase 3 MAESTRO-NASH Trial
 Phillip Leff, MD1 and Nicole E. Rich, MD, MSCS2
Phillip Leff, MD1 and Nicole E. Rich, MD, MSCS2
1Internal Medicine Resident, Division of Internal Medicine, Creighton University School of Medicine, Phoenix, Arizona.
2Assistant Professor, Associate Director of the Liver Tumor Program, Harold C. Simmons Comprehensive Cancer Center, Associate Director of Clinical Research, Division of Digestive and Liver Diseases, UT Southwestern Medical Center, Dallas Texas.
This summary reviews Harrison SA, Bedossa P, Guy CD et al. A phase 3, randomized controlled trial of resmetirom in NASH with liver fibrosis. NEJM 2024; 390(6): 497-509.
Access the article through PubMed
Correspondence to Nicole Rich, MD. Associate Editor. Email: EBGI@gi.org
Keywords: Steatotic liver disease, liver fibrosis, metabolic associated liver disease, clinical trials
STRUCTURED ABSTRACT
Question: Does the oral thyroid hormone receptor beta-selective agonist resmetirom decrease fibrosis and produce resolution of nonalcoholic steatohepatitis (NASH; now also known as metabolic-dysfunction associated steatohepatitis [MASH]) with fibrosis?
Design: Multicenter, phase 3, double-blind, randomized, placebo-controlled clinical trial.
Setting: Two hundred and forty-five centers across 15 countries (United States, Australia, Austria, Belgium, Canada, France, Germany, Hungary, Israel, Italy, Mexico, Poland, Spain, Switzerland, and the United Kingdom) between March 2019 and July 2021.
Patients: Adults aged >18 years with metabolic syndrome and biopsy-confirmed NASH. Screening biopsies were performed within 6 months of randomization, and participants were required to have a nonalcoholic fatty liver disease (NAFLD) activity score (NAS) >4 and fibrosis stage ranging from stage F1B to F3. At least 50% of the total enrollment was required to have fibrosis stage F3. Participants were also required to have stable weight (<5% change in 3 months) with stable doses of glucagon-like peptide-1 (GLP-1) agonists for >6 months prior to biopsy, if applicable. Exclusion criteria included: 1) alcohol consumption (>30 g/day for men, >20 g/day for women), 2) hemoglobin (Hgb) A1c >9%, 3) presence of other, concomitant chronic liver disease, and 4) fibrosis stage F0 (no fibrosis) or F4 (cirrhosis).
Interventions: Participants randomized 1:1:1 to 1 of 3 study arms: 1) resmetirom 80 mg once daily, 2) resmetirom 100 mg once daily, or 3) placebo with stratification for presence of type 2 diabetes mellitus (DM) and fibrosis stage (F1, F2, F3). All participants received nutrition and exercise counseling according to current recommendations. A second liver biopsy was performed at 52 weeks.
Outcomes: Dual primary endpoints were assessed at week 52, including: 1) NASH resolution, defined as ballooning score of 0, lobular inflammation score of 0 or 1, and reduction in NAS by >2 points with no worsening of fibrosis and 2) Improvement in fibrosis by at least 1 stage with no worsening of NAS. Outcomes were assessed by central, independent review by 2 pathologists. A secondary end point was percent change in baseline low-density lipoprotein (LDL) cholesterol at week 24.
Data Analysis: Intention-to-treat analysis using Cochran-Mantel-Haenszel test.
Funding: Madrigal Pharmaceuticals (West Conshohocken, PA) manufacturer of resmetirom.
Results: Nine hundred and fifty-five patients were randomized: mean age was 56.6 years, mean body mass index (BMI) was 35.7, 89% White, and most had metabolic risk factors (78% hypertension, 71% dyslipidemia, and 67% type 2 diabetes). Most patients (60%) had F3 fibrosis, with 33% having F2 fibrosis and only 5% having F1B fibrosis.
For NASH resolution with no worsening of fibrosis, resmetirom 80mg and resmetirom 100 mg was superior to placebo: 25.9% and 29.9% vs 9.7%, respectively, P<0.001 for both comparisons). For decrease in fibrosis score by at least 1 with no worsening of NAFLD activity score, resmetirom 80mg and resmetirom 100mg were also superior to placebo: 24.2% and 25.9% vs 14.2%, respectively, P<0.001 for both comparisons (Figure 1). For both NASH resolution and fibrosis improvement by ≥1 stage, resmetirom 80 mg and resmetirom 100mg was superior to placebo: 14.2% and 16% in the 100 mg vs 4.9%, respectively, P<0.001 for both comparisons.
Beneficial effects on LDL cholesterol levels were observed in both intervention groups at week 24 (-13.6% in the 80 mg resmetirom group and -16.3% in the 100 mg resmetirom group) but not in the placebo group (0.1%; P<0.001 for both comparisons). Additionally, larger decreases in levels of other atherogenic lipids and lipoproteins (e.g., triglycerides, non-high density lipoprotein (HDL) cholesterol, apolipoprotein B) compared to baseline were observed in the resmetirom groups compared to placebo.
Most adverse events (AEs) were mild or moderate, with diarrhea (27.0% and 33.4% vs 15.6%, respectively) and nausea (22.0% and 18.9% vs 12.5%, respectively) occurring more commonly in the resmetirom 80 mg and 100 mg groups compared to placebo. Diarrhea was generally self-limited with duration. However, AEs led to trial discontinuation in more patients in the 100 mg resmetirom group (6.8%) compared to those in the 80 mg group (1.8%) and those in the placebo group (2.2%).
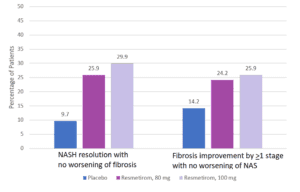
Figure 1. Percentage of patients reaching primary end points at week 52. Placebo N= 318; resmetirom, 80 mg N= 316; resmetirom, 100 mg N=321. NAS, nonalcoholic fatty liver disease activity score. NASH, nonalcoholic steatohepatitis.
COMMENTARY
Why Is This Important?
NAFLD, recently renamed metabolic-dysfunction associated liver disease (MASLD),1 is highly prevalent, affecting 30% of the global population.2 It is the fastest rising cause of hepatocellular carcinoma (HCC)3, 4 and the most rapidly increasing indication for liver transplant in the US.5 MASLD encompasses a spectrum of disease, from simple steatosis (i.e., excess fat accumulation in hepatocytes) to its more severe form, MASH, characterized by hepatocyte ballooning, inflammation, and progressive fibrosis.1 An estimated 25% of patients with MASH will eventually develop cirrhosis, but fibrosis progression is incompletely understood and varies significantly between patients.6 Fibrosis stage is the most important predictor of all-cause mortality, liver-related events and cardiovascular disease in MASLD.7 Liver biopsy remains the gold standard for diagnosis and to assess disease severity (evaluated with the NAS) and stage (fibrosis), but is not routinely performed in clinical practice given its invasiveness.
The pathophysiology of MASH is complex with several potential therapeutic targets.8 Despite an active research landscape and promising novel pharmacologic agents, none had shown safety and efficacy in phase 3 trials to date. As fibrosis stage is the key driver of clinical outcomes and survival in MASH, the US Food and Drug Administration (FDA) endpoints for late-stage MASH trials have focused on improvement in histology (that is, MASH resolution without worsening fibrosis or improvement of fibrosis stage without worsening MASH), resulting in at least 2 liver biopsies being required (at entry and end of treatment).9 Given the lack of FDA-approved drugs for MASH, treatment has relied on lifestyle modifications (i.e., diet and aerobic exercise) and weight loss with varying efficacy.10
Resmetirom, an oral selective thyroid hormone receptor beta (THR-b) agonist, is the first investigational drug to achieve both fibrosis improvement and NASH resolution in a phase 3 trial.11 Data from the landmark MAESTRO-NASH trial have led to resmetirom becoming the first FDA-approved therapy for the treatment of patients with non-cirrhotic MASH with moderate to advanced fibrosis (i.e., stage F2-F3 fibrosis) in March 2024.
Key Study Findings
Results favoring resmetirom were consistent across key subgroups; further, changes in lipid profiles, liver biochemistries, and non-invasive steatosis and fibrosis assessments all favored resmetirom. Resmetirom appears to be safe and well-tolerated, with most common adverse events being self-limited diarrhea and nausea at treatment initiation; serious adverse events were similar across all 3 arms, including the placebo arm (10.9% to 12.7%).
Caution
The primary limitation of MAESTRO-NASH to date is the lack of clinical outcomes data, as both primary endpoints were histologic and assessed at 52 weeks from baseline. Long-term safety and durability of histologic response beyond 52 weeks have also yet to be assessed. However, the trial is planned to continue for a total 54 months of treatment to accrue and evaluate potential benefits, including all-cause mortality and liver-related clinical outcomes (i.e., progression to cirrhosis, hepatic decompensation, need for liver transplantation). Additionally, results from this trial may not be generalizable to all populations, including Black patients and those with an overlap of MASH and alcohol-related liver disease (i.e., those classified within the newly termed metALD group, which encompasses a spectrum across which the relative contribution of MASLD and ALD varies). It should be noted that this trial was published shortly after new nomenclature for steatotic liver disease was endorsed by the American Association for the Study of Liver Diseases (AASLD) and other professional societies.1 It also remains unclear whether patients with cirrhosis and those that have not yet developed fibrosis (stage F0) may benefit from resmetirom.
My Practice
As a hepatologist, it is incredibly exciting to (finally!) see the first FDA-approved medication for MASH, a disease impacting many of our patients. Identifying the subset of patients most likely to benefit from resmetirom (and anticipated future NASH therapies) will be the next challenge facing both subspecialty and primary care clinicians. Gastroenterology and hepatology clinics alone lack the capacity to diagnose and risk stratify the entire large population of patients with MASLD. Provider education and proposed primary care pathways will be critical to risk stratify patents and minimize the number of patients requiring biopsy.
The 2023 AASLD clinical practice guidance recommends an algorithm wherein patients at higher risk for advanced fibrosis due to MASH (i.e., patients with 2 or more metabolic risk factors, particularly those with pre-diabetes or diabetes) are screened with FIB-4 testing every 1-2 years.12, 13 Patients with moderate or high risk based on FIB-4 are recommended to undergo second-line testing like vibration-controlled transient elastography or enhanced liver fibrosis testing, and, if still consistent with moderate or higher risk of fibrosis, the patient is referred to a subspecialist for possible intervention. As non-invasive tests have excellent negative predictive value, patients identified to be low riskcan be managed in primary care. We have begun to implement this care pathway at our health system.
It is not feasible to biopsy the entire population of patients with MASH and suspected fibrosis. While non-invasive testing is useful to rule in or rule out patients with severe disease, liver biopsy remains the gold standard for grading MASH severity and staging fibrosis, it is not without limitations including sampling variability, reader variation and safety. These limitations and access become a greater concern when considering the need to monitor treatment response. Fortunately, the FDA-approved label does not include a requirement for biopsy to diagnose moderate-to-severe fibrosis, and most clinicians have access to some sort of non-invasive testing.
Given the close correlation between MASLD and metabolic syndrome and diabetes, it will also be important to continue to counsel patients on lifestyle modifications for healthy weight loss, consider pharmacologic or surgical therapies for treatment of obesity, and cardiac risk factor modification.
For Future Research
Given the sheer number of patients with MASH that may potentially benefit from resmetirom (and anticipated future novel therapeutics), there is an urgent need to refine patient care pathways and implement strategies to identify patients at greatest risk of MASH progression and adverse clinical outcomes. As the leading causes of death among patients with MASLD are still cardiovascular events and extra-hepatic malignancies,14 the benefits and risks of long-term therapy must be considered in patients with low-risk NASH and those with concomitant severe comorbidities and limited life expectancy. Cost-effectiveness studies that consider competing risks (e.g., comorbidities, liver transplantation) are needed to estimate the expected burden of long-term therapy on healthcare systems. Proactive strategies and interventions to address individual out-of-pocket costs and provide equitable access to new, high-cost therapeutics will be critical to prevent widening of existing racial, ethnic, and socioeconomic disparities in MASH severity and outcomes.15
Finally, there remains an unmet need for non-invasive tests to monitor treatment response and assess risk of important clinical outcomes, including progression to cirrhosis, liver decompensation, development of HCC and mortality. While there are several promising serum- and imaging-based biomarkers, most are limited regarding positive predictive value and require further validation in diverse patient populations and practice settings. As the landscape of therapeutics for MASH continues to evolve, accurate and widely available tools for non-invasive risk stratification and monitoring of treatment response will only become more crucial.
Conflicts of Interest
Dr. Rich has served as consultant or on advisory boards for AstraZeneca, Eisai, Exelixis and Genentech, unrelated to the present work. Dr. Leff reports no conflicts of interest.
REFERENCES
- Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023;78:1966-1986.
- Younossi ZM, Golabi P, Paik JM, et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023;77:1335-1347.
- Huang DQ, Singal AG, Kono Y, et al. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab 2022;34:969-977.e2.
- Rich NE. Changing epidemiology of hepatocellular carcinoma within the United States and worldwide. Surg Oncol Clin N Am 2024;33:1-12.
- Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol 2021;19:580-589.e5.
- Zhai M, Liu Z, Long J, et al. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Scientific Reports 2021;11:5195.
- Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149:389-97.e10.
- Parlati L, Régnier M, Guillou H, et al. New targets for NAFLD. JHEP Rep 2021;3:100346.
- Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis.__Hepatology 2011; 54:344-53.
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149:367-78.e5; quiz e14-5.
- Harrison SA, Bedossa P, Guy CD, et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N Engl J Med 2024;390:497-509.
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77.
- Kanwal F, Shubrook JH, Adams LA, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021;161:1657-1669.
- Konyn P, Ahmed A, Kim D. Causes and risk profiles of mortality among individuals with nonalcoholic fatty liver disease. Clin Mol Hepatol 2023;29:S43-s57.
- Rich NE, Oji S, Mufti AR, et al. Racial and ethnic disparities in nonalcoholic fatty liver disease prevalence, severity, and outcomes in the United States: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16: 198-210.e2.

