Ustekinumab and Vedolizumab Demonstrate Safety During Pregnancy-Updates From the PIANO Registry

Rahul Dalal, MD, MPH
Instructor, Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
This summary reviews Chugh R, Long MD, Jiang Y, et al. Maternal and neonatal outcomes in vedolizumab and ustekinumab exposed pregnancies: Results from the PIANO registry. Am J Gastroenterol. 2024; 119(3):468-476.
Access the article through The American Journal of Gastroenterology
Correspondence to Rahul Dalal, MD, MPH, Associate Editor. Email: EBGI@gi.org
Keywords: Ustekinumab, vedolizumab, pregnancy, PIANO
STRUCTURED ABSTRACT
Question: Are ustekinumab, an interleukin (IL)-23 monoclonal antibody, and vedolizumab, an anti-integrin monoclonal antibody, safe during pregnancy in patients with inflammatory bowel disease (IBD)?
Design: This multicenter, prospective observational study included pregnant women with singleton pregnancies and a diagnosis of IBD, and is otherwise known as the “Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes” (PIANO) study. This article provides an updated analysis through November 2022 and specifically focuses on pregnant women exposed to ustekinumab and vedolizumab. Patients were administered questionnaires at enrollment, each trimester, delivery, and 4, 9, and 12 months post-delivery (Figure 1).
Setting: The PIANO registry has enrolled patients from more than 30 centers in the United States since 2007.
Patients: Through November 2022, 1,669 completed singleton pregnancies with 1,610 resulting in the birth of a baby were included. Spontaneous abortion or miscarriage occurred in 2.5% of patients. Among these pregnant women, 47 were exposed to ustekinumab and 66 were exposed to vedolizumab.
Exposures: IBD medications, including ustekinumab, vedolizumab, anti-tumor necrosis factor (TNF) agents, immunomodulators, combination anti-TNF/thiopurine therapy, and no exposure to biologics or thiopurines.
Outcomes: Primary outcomes included congenital malformations, spontaneous abortion, preterm birth, Cesarean section (C-section), small for gestational age, low birth weight, neonatal intensive care unit (NICU) stay, intrauterine growth restriction, placental events, neonatal/infant infections, and infant developmental milestones.
Data Analysis: Bivariate analyses were utilized to determine the association of biologic therapy class with pregnancy and neonatal outcomes. Fisher’s exact test and Kruskal-Wallis test were also used to compare categorical and continuous data.
Funding: The Crohn’s and Colitis Foundation and the Leona M. and Harry B. Helmsley Charitable Trust.
Results: Among the 1,669 pregnancies in the registry, demographic data included mean maternal age: 32; IBD duration: 8.5 years; disease type: 62% Crohn’s disease, 35% ulcerative colitis, 2% IBD unclassified; mean total pregnancies, including current: 2.1; IBD drug exposure during pregnancy: anti-TNF: 43%; immunomodulator: 14%; combination of anti-TNF/immunomodulator: 11%; no IBD drug exposure: 26%; ustekinumab: 3%; and vedolizumab: 4%.
There was no observed increased risk in congenital malformations, spontaneous abortion, small for gestational age, low birth weight, NICU stay, placental complications, or intrauterine growth restriction with either vedolizumab or ustekinumab compared to other therapy exposures. Ustekinumab was associated with a lower rate of pre-term birth compared to all other therapies, including vedolizumab. Rates of serious infections in infants within 12 months were similar between all groups, though non-serious infections were less common for ustekinumab.
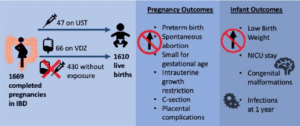
Figure 1. Visual abstract showing study design and pregnancy/infant outcomes. Abbreviations: IBD, inflammatory bowel disease; NICU, neonatal intensive care unit; PIANO, Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes
COMMENTARY
Why Is This Important?
Prior data from the PIANO registry demonstrated that corticosteroid use in IBD patients during pregnancy was associated with increased adverse maternal and fetal outcomes, confirming the importance of achieving and maintaining IBD remission prior to pregnancy. Further data from the PIANO registry confirmed the safety of anti-TNF agents and thiopurines during pregnancy, demonstrating no increased risk of spontaneous abortion/miscarriage, congenital malformations, or infant infections.2 Until this updated analysis, prior studies reporting pregnancy outcomes with vedolizumab and ustekinumab exposure during pregnancy were limited due to small sample sizes.3-4
This prospective study by Chugh et al is the largest study to assess maternal and infant outcomes after exposure to ustekinumab or vedolizumab while also providing comparators of IBD patients with no IBD drug exposure and IBD patients treated with anti-TNF agents or immunomodulators. Since there was no increased risk of adverse pregnancy or infant outcomes after exposure to ustekinumab or vedolizumab, these data provide critical reassurance to both patients and providers who are either considering these therapies or already receiving them near the time of pregnancy. Patients can be counseled that these biologics, like anti-TNFs, should be continued and uninterrupted during pregnancy with no expectation for an increased risk of adverse outcomes.
Key Study Findings
Ustekinumab exposure was associated with a lower risk of pre-term birth when compared to other therapies, though this potential benefit needs to be confirmed in larger cohorts.
Caution
This was a relatively small study with only 113 patients exposed to either ustekinumab or vedolizumab. This could lead to type 2 error, i.e., failure to observe significant rates of adverse events that could be identified in larger cohorts. Additionally, the findings of this study are based on patient questionnaires, which are subject to selection, sampling, and recall bias.
My Practice
In my practice, I now counsel patients that they should continue ustekinumab or vedolizumab during pregnancy if these agents had been controlling their disease well prior to pregnancy. Additionally, I now encourage these agents (when clinically appropriate) as options in women who are contemplating pregnancy and need to initiate biologic therapy for either Crohn’s disease or ulcerative colitis. I also emphasize important findings from other studies of the PIANO registry, particularly that flares of disease and use of corticosteroids are associated with adverse perinatal outcomes.1,5 This underscores the importance of continuing anti-TNF agents, ustekinumab, and vedolizumab during pregnancy to maintain remission of IBD.
For Future Research
Larger, prospective cohorts are needed to confirm the findings of this study. Additionally, research is needed regarding the comparative effectiveness and safety of other biologic therapies, including sphingosine-1-receptor modulators and JAK1 inhibitors, to maintain remission of IBD during pregnancy without any increase in adverse pregnancy or neonatal outcomes.
Conflict of Interest
Dr. Dalal has research grant support from Janssen and Pfizer and has served as a consultant for Janssen, Takeda, and Centaur Labs.
Rishika Chugh@MLongMD
Millie Long
@UmaMahadevanIBD
Uma Mahadevan
REFERENCES
- Schoenfeld P, Charabaty A. Achieve IBD remission before pregnancy: PIANO registry data shows adverse perinatal outcomes for infants associated with IBD flares and steroid use. Evidence-Based GI 2022. https://gi.org/journals-publications/ebgi/schoenfeld_september_2022/
- Mahadevan U, Long MD, Kane SV, et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology 2021;160(4):1131-1139.
- Mitrova K, Pipek B, Bortlik M, et al. Safety of ustekinumab and vedolizumab during pregnancy-pregnancy, neonatal, and infant outcome: A prospective multicentre study. J Crohns Colitis 2022;16(12):1808-1815.
- Gisbert JP, Chaparro M. Safety of new biologics (vedolizumab and ustekinumab) and small molecules (tofacitinib) during pregnancy: A review. Drugs 2020;80(11):1085-1100.
- Odufalu FD, Long M, Lin K, Mahadevan U. Exposure to corticosteroids in pregnancy is associated with adverse perinatal outcomes among infants of mothers with inflammatory bowel disease: results from the PIANO registry. Gut 2022;71(9):1766-1772.

