Mirikizumab Is Effective for Induction and Maintenance of Ulcerative Colitis
Rahul Dalal, MD, MPH
Instructor, Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
This summary reviews D’Haens G, Dubinsky M, Kobayashi T, et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2023 ;388(26):2444-2455. Erratum in: N Engl J Med. 2023 Aug 24;389(8):772.
Access the article through PubMed
Correspondence to Rahul Dalal, MD, MPH, Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Is mirikizumab (Omvoh; Eli Lilly, Indianapolis, IN), a monoclonal antibody directed at the p19 subunit of interleukin-23, a proinflammatory factor, superior to placebo for induction and maintenance of clinical remission for moderate-to-severe ulcerative colitis (UC)?
Design: LUCENT-1 and LUCENT-2 were phase 3, randomized, double-blind, placebo-controlled trials (RCTs) of mirikizumab for moderate to severe UC. In the 12-week induction trial (LUCENT-1), patients were randomized 3:1 (mirikizumab: placebo). Patients who responded to mirikizumab induction were included in the 40-week maintenance trial (LUCENT-2), in which patients were randomized 2:1 (mirikizumab:placebo).
Setting: LUCENT-1 included 383 centers in 34 countries, and LUCENT-2 included 367 centers in 34 countries.
Patients: Inclusion criteria included: 18-80 years old; moderate-to-severe ulcerative colitis based on modified Mayo score >4 (0-9 scale); prior history of inadequate response, loss of response or intolerant of conventional therapy (glucocorticoids or immunomodulators) or biologic therapy. Exclusion criteria included: prior exposure to anti-interleukin (IL)-12 or anti-IL 23 monoclonal antibodies or prior treatment failure with >3 different biologic therapies. Note that the modified Mayo Score assesses rectal bleeding score (0-3), stool frequency score (0-3), endoscopy subscore (0-3), so the score range is 0-9 with 9 representing most severe UC.
Interventions: In the 12-week induction RCT (LUCENT-1), mirikizumab 300 mg intravenously (IV) or placebo IV every 4 weeks for 12 weeks. In the subsequent 40-week maintenance of remission RCT (LUCENT-2), mirikizumab 200 mg or placebo subcutaneously every 4 weeks.
Outcomes: Primary endpoints were clinical remission at week 12 for LUCENT-1 and clinical remission at week 40 (week 52 overall) for LUCENT-2. Clinical remission was defined as stool frequency subscore of 0-1, rectal bleeding subscore of 0, and an endoscopic subscore of 0-1, based on the modified Mayo Score. Secondary endpoints included clinical response, endoscopic response, and improvement in bowel movement urgency, which was assessed with the Urgency Numeric Rating Scale (Urgency NRS), a newly validated scale from 0 (no urgency) to 10 (worst possible urgency). Safety events were also assessed.*
Data Analysis: Primary and secondary endpoints were analyzed in modified intention-to-treat populations, including all patients who underwent randomization and received any amount of mirikizumab or placebo (but excluding those who were affected by an electronic clinical outcomes transcription error). Safety events were assessed in all patients who underwent randomization and received any amount of mirikizumab or placebo (including those affected by the transcription error). Binary endpoints were assessed using the Cochran-Mantel-Haenszel test with adjustment for stratification factors. Continuous endpoints were compared using mixed-effects models with repeated measures analysis. *
Funding: Eli Lilly, manufacturer of mirikizumab.
Results: A total of 1,281 patients were randomized for the 12-week induction of remission RCT (LUCENT-1): mean age 41-43; male sex 56%-61%; disease duration 6.9-7.2 years; left sided colitis 63%-64%; previous treatment failure with biologics 40%-42% or inadequate response to biologics 23%-24%. A significantly higher percentage of patients receiving mirikizumab achieved clinical remission at week 12 vs placebo (24.2% vs 13.3%, respectively). Clinical response, endoscopic remission, bowel urgency, and steroid-free remission were also significantly more common with mirikizumab compared to placebo (Figure 1). A total of 544 with response to mirikizumab were randomized for the 40-week maintenance of remission RCT (LUCENT-2), and a significantly higher percentage of patients receiving mirikizumab maintained/achieved clinical remission at week 40 vs placebo (49.9% vs 25.1%, respectively) and achieved secondary endpoints (Figure 2).
Incidence of adverse events were similar in the mirikizumab and placebo group. There were 15 opportunistic infections (including 6 herpes zoster) and 8 diagnoses of cancer in the mirikizumab group compared to 1 opportunistic infection (herpes zoster) and no diagnoses of cancer in the placebo group.*
*Although these trials used a classic double-blind, placebo-controlled, randomized study design with modified intention-to-treat analysis, study methodology and results are too detailed to summarize comprehensively. Readers are encouraged to review the full study publication.
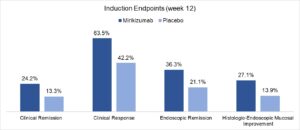
Figure 1. LUCENT-1. 12-week induction of remission randomized controlled trial.

Figure 2. LUCENT-2. 40-week maintenance of remission randomized controlled trial.
COMMENTARY
Why Is This Important?
Despite an increasing number of approved therapies for moderate-to-severe UC, many patients will fail to achieve adequate response or lose response to therapy over time.1 Additionally, the use of many treatments is limited due to the risk of infection and malignancy. The IL-23 pathway has become an important pathway to balance the effective treatment of autoimmune disease while minimizing adverse effects. Ustekinumab (Stelara; Janssen Pharmaceuticals, Beerse, Belgium) was the first drug approved for both Crohn’s disease and UC that targets this pathway, specifically blocking the shared p40 subunit of IL-12 and IL-23,2,3 while mirikizumab is the first drug approved for UC that selectively blocks the p19 subunit of IL-23.
The study assessed typical endpoints such as clinical remission, clinical response, and endoscopic remission. Other endpoints reflecting modern treatment goals in UC were also assessed. Mirikizumab was effective for these endpoints, including histologic-endoscopic mucosal remission and resolution of bowel urgency, which is increasingly recognized as the most important symptom for many patients with UC.4 The LUCENT-1 and LUCENT-2 trials are unique as the first phase 3 RCTs to utilize the newly validated Urgency NRS to evaluate this endpoint. Given its effectiveness and favorable safety profile, mirikizumab will serve as another reasonable treatment consideration for patients with moderate-to-severe UC.
It’s been hypothesized that the selective blockade of the p19 subunit of IL-23 would produce better outcomes in IBD than blockade of the shared p40 subunit of IL-12 and IL-23. Risankizumab (Skyrizi; AbbVie Pharmaceuticals, Chicago, IL), which also selectively blocks the p19 subunit and has been approved for Crohn’s disease, did demonstrate superiority vs ustekinumab for endoscopic and clinical remission of Crohn’s disease in the SEQUENCE trial.5 Thus, future comparative trials of ustekinumab vs mirikizumab in UC will be beneficial.
Key Study Findings
Caution
There were numerically more opportunistic infections and cancer in the mirikizumab group compared to placebo. Long-term data is needed to better understand the safety of mirikizumab, particularly in relation to other agents targeting the IL-23 pathway (e.g. ustekinumab).
My Practice
At this time, there is insufficient comparative data for me to routinely use mirikizumab over ustekinumab, and ustekinumab may be more convenient for my patients since it has a less frequent maintenance dosing schedule (every 8 weeks). As always, insurance coverage will also play a role in my prescribing. I may also consider mirikizumab in individuals who had secondary loss of response to ustekinumab and have also exhausted other classes of therapy.
For Future Research
Future research is needed to compare the effectiveness and safety of mirikizumab to ustekinumab and other classes of therapy (e.g. vedolizumab, Janus kinase inhibitors) to determine the optimal positioning of this agent for patients with moderate-to-severe UC.
Conflicts of Interest
Dr. Dalal has received grant support from Janssen Pharmaceuticals and Pfizer Pharmaceuticals and has served as a consultant for Centaur Labs.
REFERENCES
- Raine T, Danese S. Breaking through the therapeutic ceiling: What will it take? Gastroenterology 2022;162(5):1507-1511.
- Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012;367(16):1519-28.
- Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381(13):1201-1214.
- Dubinsky MC, Irving PM, Panaccione R, et al. Incorporating patient experience into drug development for ulcerative colitis: development of the urgency numeric rating scale, a patient-reported outcome measure to assess bowel urgency in adults. J Patient Rep Outcomes 01 2022;6(1):31.
- Peyrin-Biroulet L, Chapman JC, Colomber JF, et al. Risankizumab versus ustekinumab for patients with moderate to severe Crohn’s disease: results from the Phase 3b SEQUENCE study. United European Gastroenterol J 2023;11: 919-940. Abstract LB01.


