Tenapanor (IBSRELA) for Treatment of IBS-C: Effective Over 26 Weeks
Philip Schoenfeld, MD, MSEd, MSc (Epi)
Chief (Emeritus)-Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI
This article reviews Chey WD, Lembo A, Yang Y, Rosenbaum DP. Efficacy of Tenapanor in Treating Patients With Irritable Bowel Syndrome with Constipation: A 26-Week, Placebo-Controlled Phase 3 Trial (T3MPO-2). Am J Gastroenterol 2021; 116: 1294-1303.
Correspondence to Philip Schoenfeld, MD, MSEd, MSc (Epi), Editor-in-Chief. Email: EBGI@gi.org
Access the Article in The American Journal of Gastroenterology
Question: Is tenapanor (IBSRELA), a first-in-class, small-molecule inhibitor of the GI sodium/hydrogen exchanger isoform 3 (NHE3) superior to placebo in patients with inflammatory bowel syndrome with constipation (IBS-C) for improving global IBS-C symptoms, abdominal discomfort, and complete spontaneous bowel movements (CSBM) based on FDA-defined responder endpoints?
Funding: Ardelyx Pharmaceuticals.
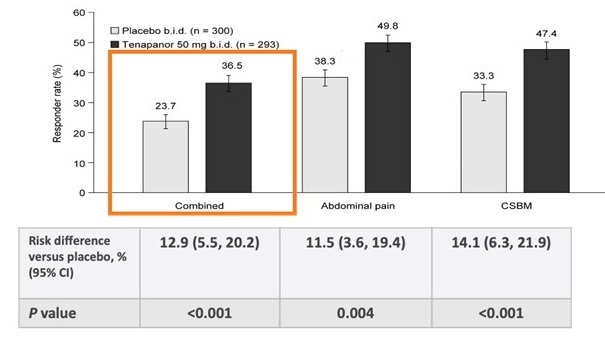
Figure 1. > 6/12 week responders for FDA-combined endpoint, abdominal pain endpoint, and CSBM endpoint.
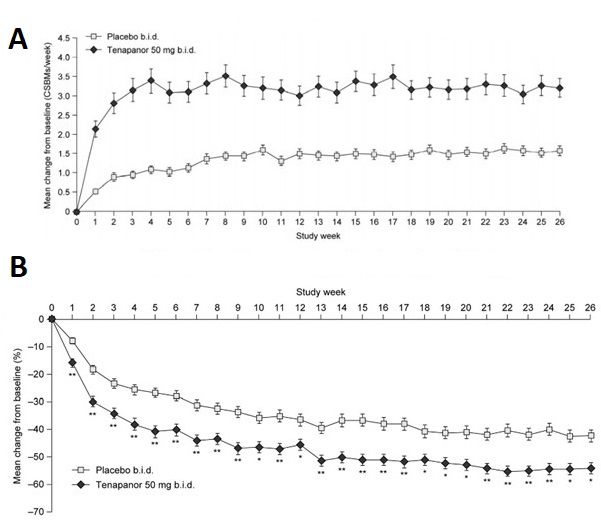
Figure 2. Weekly change in CSBMs (A) and abdominal pain (B).
COMMENTARY
Why Is This Important?
Although IBS is commonly characterized by altered intestinal motility and visceral hypersensitivity, the underlying pathophysiology is complex and may involve defective brain-gut interactions, alterations in gut flora, genetic predispositions, and defects in enteric nervous system functioning, among others. Thus, it’s unsurprising that even the most effective IBS treatments rarely demonstrate efficacy in more than 50% of patients1. Therefore, it’s heartening that we’re getting more treatment options for IBS-C. Tenapanor (IBSRELA) is the first sodium/hydrogen exchanger 3 (NHE3) inhibitor approved for use. NHE3 is expressed on the surface of the intestine, and tenapanor inhibits dietary sodium absorption by inhibiting NHE32. Animal studies demonstrate that it also decreases intestinal permeability and visceral hypersensitivity, although the mechanism for these actions is unclear since the pharmacologic action primarily blocks sodium absorption2.
Key Study Findings
Tenapanor is clearly superior to placebo for improvement in abdominal discomfort, stool frequency and stool consistency.
Some practitioners might be disappointed that only 36.5% of patients were “responders,” but the complicated patient-reported outcomes required by the FDA are a high threshold for “success” and don’t necessarily translate well to clinical care. It’s notable that tenapanor-treated patients improved from mean of 0.1 CSBMs/week to more than 3 CSBMs/week, which was consistent through 26 weeks as well as achieving approximately 50% reduction in abdominal pain from baseline (Figure 2).
Caution
With respect to study design, this is an excellent clinical trial that meets all FDA-requirements for a Phase III RCT. Based on baseline characteristics (e.g., average of 0.1 CSBMs/week), study patients had fairly severe IBS-C symptoms, which might impact generalizability of study results. Since tenapanor works at the surface of the colonic mucosa, it’s considered “minimally absorbed”. This is consistent with finding no drug-drug interactions and no difference in adverse events between tenapanor- and placebo-treated patients with the exception of diarrhea.2 As seen with most effective treatments for IBS-C, a minority of patients will experience diarrhea. Diarrhea was more commonly reported by tenapanor-treated patients vs placebo-treated patients (16.0% vs 3.7%), although discontinuation of study medication due to diarrhea only occurred in 6.5% of tenapanor-treated patients.
My Practice
Per the ACG Guideline on Management of IBS1, guanylate cyclase-C agonists (i.e., linaclotide and plecanatide) are the only treatments that receive a strong recommendation based on high quality RCT evidence and they are the cornerstone of my treatment for IBS-C. Although I recognize that many practitioners may prefer to start IBS-C treatment with an osmotic laxative, it’s worth remembering that the ACG Guideline suggests against using polyethylene-glycol products (e.g., MiraLax) to relieve global IBS symptoms in IBS-C since RCTs report no significant differences versus placebo for improvement in abdominal discomfort symptoms. Regardless, when patients are referred to me, they have invariably already tried osmotic laxatives, and most patients will have tried and failed these over-the-counter medications prior to seeing a gastroenterologist for IBS-C1. For patients who don’t get adequate relief with linaclotide or plecanatide, I’ll try tenapanor. Since tenapanor has a unique mechanism of action, I won’t wait for patients to fail trials of linaclotide AND plecanatide. I’ll simply switch to tenapanor after a patient fails their initial course of a guanylate cyclase-C agonist.
Consistent with the study findings, I’ll emphasize to patients that it may take 8-12 weeks to achieve optimal decrease in abdominal discomfort symptoms and encourage patients to continue treatment even if there is only mild improvement in the first 1-2 weeks. I’ll also proactively educate my patients that loose stools may occur in the first week of treatment, since this is when tenapanor-associated diarrhea is most likely to occur. Since I treat more severe IBS-C patients, I frequently combine therapies. I doubt that I’d combine tenapanor with a guanylate cyclase-C agonist, but I will combine it with peppermint oil capsules as an on-demand or daily anti-spasmodic treatment or a neuromodulator, such as duloxetine (Cymbalta) at 30-60mg daily, and/or referral to our dietitician for instruction in low-FODMAP diets.
For Future Research
Comparative statements about the efficacy of tenapanor (IBSRELA) compared to other IBS-C treatments can’t be made in the absence of head-to-head trials. It would be optimal, albeit unlikely, to see these types of trials in the future. Also, RCT data about the efficacy of combination therapy (e.g., tenapanor plus neuromodulator) would be helpful. Based on my clinical experience, many patients will experiment by only using tenapanor 50 mg daily or even as a prn medication. Real-world data about this would be instructive.
Conflict of Interest
Dr. Schoenfeld reports that he sits on advisory boards and speakers bureaus, and is a consultant for Ironwood Pharmaceuticals, AbbVie Pharmaceuticals, and Salix Pharmaceuticals. He also serves as an advisory board member for Takeda Pharmaceuticals, Ardelyx Pharmaceuticals, and Phathom Pharmaceuticals.
REFERENCES
1. Lacy BE, Pimentel M, Brenner D, et al. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol 2021; 116: 17-44.
2. IBSRELA (Tenapanor) Prescribing Information. www.accessdata.fda.gov/drugsatfda_docs/label/2019/211801s000lbl Accessed June 11, 2022.


