Making Bowel Preparation Palatable: Efficacy of a Novel Sports Drink Flavor-Optimized PEG and Sulfate Bowel Preparation
 Ahmad Abu-Heija, MBBS
Ahmad Abu-Heija, MBBS
Consultant Gastroenterologist, Oak Ridge Gastroenterology Associates, Oak Ridge, TN.
This summary reviews Bhandari R, Goldstein M, Mishkin DS, et al. Comparison of a novel, flavor-optimized, polyethylene glycol and sulfate bowel preparation with oral sulfate solution in adults undergoing colonoscopy. J Clin Gastroenterol; 2023;57(9):920-927.
Access the article through PubMed
Correspondence to Ahmad Abu-Heija, MBBS, Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: In patients undergoing a colonoscopy, does a flavored polyethylene glycol (PEG) and sulfate solution (FPSS) that is optimized to taste like a sports drink (SUFLAVE; Braintree Laboratories, Braintree, MA) offer better tolerability with similar bowel cleansing to a well-established, US Food and Drug Administration (FDA)-approved, oral sulfate salt (OSS)-based bowel preparation (SUPREP; Braintree Laboratories)?
Design: Investigator-blinded, randomized, controlled, non-inferiority study in outpatients undergoing colonoscopy for routine indications.
Setting: Thirty-two United States study sites with subjects recruited from gastroenterology practices.
Patients: A total of 500 adult subjects were randomized and 450 subjects took the preparation and were included in analysis between July 2020 and February 2021. Mean age was 56.2 years, with 58.8% female, 84.4% White. Indications included screening, polyp surveillance, GI symptoms, and inflammatory bowel disease. Patients with routine endoscopy contraindications (e.g. ileus, GI obstruction), previous significant abdominal surgeries, as well as patients with baseline electrolyte abnormalities were excluded. In addition, patients on laxatives, diuretics, and antihypertensive agents as well as patients with a history of severe renal, liver, or cardiac insufficiency were also excluded.
Intervention: The sports drink flavor-optimized FPSS solution consisted of approximately 3 L administered in a split dose with 1 L consumed the night before the procedure and 1 L again in the morning, 5-8 hours before the procedure along with 16 oz of water with each dose. The comparator group were given the standard OSS bowel preparation in a split dose with total fluid consumed amounting to 2.8 L.
Outcomes: The primary efficacy endpoints included quality of bowel cleansing using a US FDA bowel prep scoring scale which also accounts for the work of endoscopist cleansing during the exam. Cleansing was evaluated globally and segmentally using a 4-point scale, as shown in Table 1.
The primary efficacy endpoint was global cleansing. Grades of “good” or “excellent” for global cleansing of the colon were considered successful, while grades of “poor” and “fair” were considered failures. Secondary efficacy endpoints included the number (percentage) of “excellent” preparations (global score), segmental cleansing success, adequacy of cleansing and need for repreparation, adenoma detection rate (ADR), duration of colonoscopy, the volume of intraprocedural water needed to irrigate the colon, and cecal intubation rate. In addition, procedures were recorded and underwent independent blinded central reading by GI reviewers.
Subject acceptance of the prep was evaluated using a questionnaire filled by the patients when they returned for their colonoscopy after finishing the prep. Questionnaire included questions pertaining to difficulty of prep consumption, overall experience with prep comparison of this prep to previous prep, whether or not they would take the same prep again, and their rating of the aftertaste of the prep.
Data Analysis: Intention-to-treat analysis.
Funding: Braintree Laboratories, a part of Sebela Pharmaceuticals.
Results: Both preparations achieved similar global cleansing scores with high rates of cleansing success, 94% for sports drink flavor-optimized FPSS and 94% for standard OSS. This result demonstrated noninferiority between bowel preparation. Both preparations were safe and well-tolerated in the study population with no significant difference in adverse events. As for subject satisfaction, the sports drink flavor-optimized solution of PEG and sulfate solution was rated more favorably than OSS -based prep on multiple measures, including ease of consumption, overall prep experience, as well as taste (Table 2). No clinically significant differences in electrolytes were identified from baseline to date of colonoscopy for either group.

Table 1. Bowel prep scale.
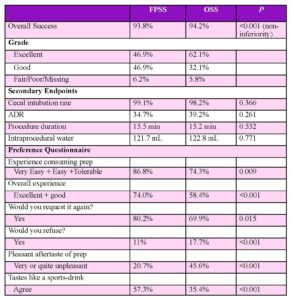
Table 2. Primary endpoint: local endoscopist cleansing ratings. Abbreviations: ADR, adenoma detection rate; FPSS, flavored polyethylene glycol and sulfate solution; OSS, oral sulfate salt bowel prep.
COMMENTARY
Why Is This Important?
An adequate bowel preparation plays an essential role in our ability to provide patients with high-quality colonoscopy. Poor bowel preparation is associated with lower ADR, reduced cecal intubation rate, prolonged procedural time, and increased risks.1,2 One of the commonly cited reasons for incomplete bowel preparation is the palatability of traditionally marketed bowel preps. Certainly, the classic 4 liter PEG-electrolyte lavage solution (ELS) (GoLytely, Braintree Laboratories, Braintree, MA) is not only large volume, but also has an unpleasant taste. This has led to widespread popularity of using 238 grams PEG-3350 (MiraLax; Bayer USA, Whippany, NJ) plus 64 ounces of a sports drink (Gatorade; Pepsico, Chicago, IL) + bisacodyl tablets. No prescription is required, and the retail cost is usually about $20-$25, while the sports drink flavoring makes it palatable. However, despite real world evidence3 that this bowel preparation is effective, it is not FDA-approved, is hypo-osmolar, and has been associated with severe hyponatremia.4
Therefore, the introduction of an FDA-approved, effective FPSS bowel preparation that is flavor-optimized to mimic a sports drink is a welcome addition for patients. Ultimately, the best bowel preparation for the patient is one that they tolerate and will consume as instructed. Otherwise, the likelihood of getting a successful colon cleansing diminishes.
Key Study Findings
Both treatment arms achieved approximately 94% successful bowel cleansing. This was done while appealing better to patients in terms of the overall experience and after-taste of the prep, with more patients noting that they would request it again as a bowel cleansing solution for future procedures.
Caution
The bowel preparation scale used in this study is different from the Boston Bowel Prep Scale, which assesses cleanliness of each bowel segment after endoscopist washing, suctioning, and cleansing of residual stool and liquid. Another limitation is the generalizability of the ADR as the studied population was a mix of screening and diagnostic procedures.
The most important limitation may be that only average-risk individuals were enrolled while most individuals at high-risk for poor bowel cleansing (e.g., prior abdominal surgery, frequent use of laxatives to treat constipation) were excluded. It’s unclear if patients with a past history of poor bowel cleansing could be enrolled. Also, since these are not osmotically-balanced solutions, patients at higher risk of electrolyte abnormalities due to renal, cardiac, or liver dysfunction were not enrolled. This limits generalizability of results.
My Practice
I’m often asked, “Any changes in the bowel prep since my last colonoscopy?” The poor palatability of regularly prescribed bowel preps is one of my patients’ most common concerns. This explains why some patients only agree to repeat colonoscopy if they can use the “MiraLax-Gatorade-bisacodyl” bowel prep. Therefore, I’ve begun to offer this PEG and OSS bowel preparation that is flavor-optimized to mimic a sports drink, especially since it’s an FDA-approved alternative that is efficacious and with a known safety profile. Cost is an issue with bowel preparations, so my nurses have downloaded coupons which promise that the patient co-pay for commercially insured patients will be no more than $50 dollars, although this is still more expensive than the over-the-counter costs of the “MiraLax-Gatorade-bisacodyl” prep.
I would not offer this to patients with multiple risk factors for colonic dysmotility (e.g., history of constipation with laxative use, diabetes mellitus, obesity, ongoing opioid use, etc.) or a history of poor bowel preparation despite adherence to bowel preparation. For these high-risk patients, I usually have patients take 6 liters of PEG-ELS as a split-prep with 4 liters on the day before the colonoscopy and 2 liters on the day of colonoscopy. If they use an osmotic laxative on a daily basis, then I may have them double the dose for 3-4 days before colonoscopy. However, I also note that combining 15 mg bisacodyl on the day before colonoscopy along with 4 liters PEG-ELS as a split-prep is the regimen with the best randomized controlled trial data supporting its efficacy in high-risk patients.5
For Future Research
Emphasis on tolerability of bowel preps is definitely a step in the right direction for achieving higher levels of bowel cleansing and as such improving outcomes. More work to evaluate the safety of this bowel preparation in patients with advanced kidney and heart disease would also provide physicians with more bowel prep options to utilize in these high-risk populations.
Conflict of Interest
Dr. Abu-Heija reports no potential conflicts of interest for this summary.
REFERENCES
- Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002;97(7):1696-700.
- Millien VO, Mansour NM. Bowel preparation for colonoscopy in 2020: a look at the past, present, and future. Curr Gastroenterol Rep 2020;22(6):28.
- Gu P, Lew D, Oh SJ, et al. Comparing the real-world effectiveness of competing colonoscopy preparations: results of a prospective trial. Am J Gastroenterol 2019; 114: 305-14.
- Schoenfeld P. Safety of MiraLax/Gatorade bowel preparation has not been established in appropriately-designed studies. Clin Gastroenterol Hepatol 2013; 11: 582.
- Schoenfeld P. It’s a bad “prep” even though the patient took it correctly: consider 15 mg bisacodyl plus 4-liter PEG split prep before next colonoscopy. Evidence-Based GI Feb 2022; 2(2): 7-12. https://gi.org/journals-publications/ebgi/schoenfeld_february2022/.

