When It’s Not Acid Reflux: Managing PPI-Resistant GERD Patients Based on Bravo Results
Ahmad Abu-Heija, MBBS,1 Philip Schoenfeld, MD, MSEd, MSc (Epi)2 and Kristle Lee Lynch, MD3

1Wayne State University, Division of Gastroenterology, Detroit, MI. 2Chief (Emeritus), Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI. 3Associate Professor of Clinical Medicine, Division of Gastroenterology, University of Pennsylvania School of Medicine, Philadelphia, PA.
This summary reviews Yadlapati R, Gyawali CP, Masihi M, et al. Optimal Wireless Reflux Monitoring Metrics to Predict Discontinuation of Proton Pump Inhibitor Therapy. Am J Gastroenterol. 2022 Oct 1;117(10):1573-1582. doi.org/10.14309/ajg.0000000000001871.
Correspondence to Philip Schoenfeld, MD, MSEd, MSc. Editor-in-Cheif. Email: EBGI@gi.org
Access this article through The American Journal of Gastroenterology
STRUCTURED ABSTRACT
Question: What metrics from wireless reflux monitoring (Bravo; Medtronic, Minneapolis, MN) predict successful discontinuation of proton pump inhibitors (PPIs) in treatment-resistant individuals with gastroesophageal reflux (GERD) symptoms (i.e., heartburn, regurgitation, and/or non-cardiac chest pain)?
Design: A double-blinded single-arm prospective trial.
Setting: Two tertiary academic centers (Northwestern University, Chicago, IL and Washington University, St Louis, MO) during 2017-2021.
Patients: A total of 132 patients completed the trial. Eligible patients were adults with significant esophageal reflux symptoms (≥2 episodes of heartburn, regurgitation, and/or noncardiac chest pain per week) who remained symptomatic despite a compliant trial of at least single-dose PPI therapy for a minimum of 8 weeks. Patients were excluded if they had endoscopic erosive GERD (Los Angeles grade C or D), long-segment Barrett’s esophagus (BE) (≥3 cm), previous foregut surgery, active heart disease, pregnancy, major motility disorder on manometry, or eosinophilic esophagitis (EoE). Patients with insufficient pH monitoring (at least 14 hours a day for ≥3 days) were also excluded. Forty percent of the patients were males, mean age 47.3 years, mean body mass index 27.1 kg/m2.
Interventions/Exposure: After being off PPI therapy for at least 1 week, patients underwent 96-hour wireless reflux monitoring using the Bravo pH probe, which was positioned 6 centimeters proximal to the squamo-columnar junction at the lower esophageal sphincter. Study patients were then instructed to refrain from resuming PPI therapy for an additional 2 weeks, although patients could use over the counter (OTC) antacids (e.g., Tums or Rolaids) up to 5 times per day. Indications to resume PPI was defined as high symptom burden with a desire to resume PPI and/or using an excess of maximal OTC antacids (i.e., more than 5 times per day). Study coordinators interviewed patients weekly to assess patients and PPI resumption. Patients, as well as study coordinators and investigators, were blinded to results of reflux testing during intervention.
Outcomes: The main outcome was inability to discontinue PPI therapy for 2 weeks.
Data Analysis: Primary analysis assessed acid exposure thresholds predictive of successful PPI discontinuation, including total, upright, supine, and daily acid exposure time (AET), defined as percent time with esophageal pH < 4.0. DeMeester score, number of reflux events, longest reflux event, symptoms reported, symptom index, and symptom association probability were also calculated.
Funding: The National Institute of Health RO1.
Results: The mean wireless reflux monitoring time was 3.4 days, and 30% of patients discontinued PPIs for the entire 3-week period. An AET threshold of 3.95% demonstrated optimal overall performance for PPI discontinuation (area under curve [AUC] 0.63 [95% confidence interval 0.52-0.73]; 75% sensitivity and 55% specificity). (Figure 1) Total AET ≤4.0% had the greatest odds of predicting PPI discontinuation (odds ratio 2.9 [1.4-6.4]) with 96-hour monitoring providing optimal AUC to predict PPI discontinuation compared to 24 hours or 48 hours of monitoring. AET > 10.0% and/or DeMeester score > 50.0 were optimally predictive of patients resuming PPI therapy.
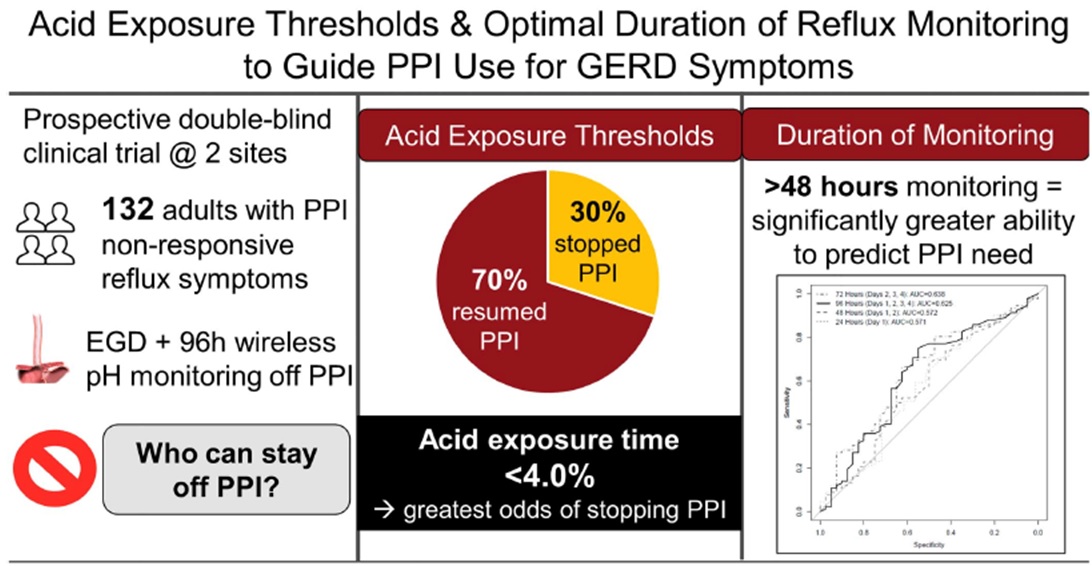
Figure 1: Study summary
COMMENTARY
Why Is This Important?
Nearly half of patients with GERD-type symptoms remain symptomatic despite PPI therapy.1 These PPI-resistant patients are quite common in GI practice and create a management conundrum, especially when esophagogastroduodenoscopy (EGD) does not demonstrate erosive esophagitis. Are the ongoing symptoms of heartburn or regurgitation or non-cardiac chest pain due to insufficient acid reduction, non-acid regurgitation, or visceral hypersensitivity of the esophagus? Which patients can and should discontinue PPIs? This is a particularly important question since many of these PPI-resistant patients are routinely prescribed twice daily dosing and may soon be prescribed vonoprazan, a potassium-competitive acid blocker, which is pharmacologically more potent, longer-acting, and has a more rapid onset than PPIs. 2-3
Although wireless reflux monitoring off PPI is a standard tool in these patients, diagnostic criteria and optimal duration of this monitoring is inadequately defined. This research by Yadlapati and colleagues demonstrates that 96-hours of monitoring is optimal for identifying patients that can successfully discontinue PPIs while setting thresholds for patients that should be able to discontinue PPI (i.e., daily and total AET < 4.0%) versus patients that are most likely PPI-resistant due to inadequate acid suppression (i.e., AET > 10.0% and/or DeMeester score > 50).
Daily and total AET ≤4.0% had the greatest odds of predicting PPI discontinuation (odds ratio 2.9 [1.4-6.4]) with 96-hour monitoring providing optimal predictive power about PPI discontinuation compared to 24 hours or 48 hours of monitoring. AET > 10.0% and/or DeMeester score > 50 were optimally predictive of patients needing to resume PPI therapy.
Caution
The primary outcome, resuming PPI therapy, was subjectively decided by the patient. A minority of patients with daily and total AET < 4.0% resumed PPIs, which may reflect anxiety or hypervigilance.
My Practice
For my patients presenting with classic symptoms of heartburn, regurgitation, and/or non-cardiac chest pain who do not have sufficient symptom relief with single-dose PPI therapy after 8 weeks, I will perform an EGD. The main purposes of this test are to assess for erosive esophagitis and/or BE, characterize the diaphragmatic hiatus, as well as rule out eosinophilic esophagitis via esophageal biopsies. If there is no evidence of Grade B+ esophagitis, BE, or EoE, I order a 96-hour wireless pH monitoring probe with the patient off of PPIs for at least 1 week.
If the daily and total AET is <4.0%, then I typically have the patient remain off of PPIs . At this point, we consider neuromodulators, diaphragmatic breathing, and/or cognitive behavioral interventions. Alternatively, if the AET is >10.0%, then the goal is to enhance reflux control. PPI optimization via timing and agent selection is first done, followed by revision or enhancement of the hiatus if symptoms persist. Vonoprazan may also be considered after it becomes available as expected later this year.
For Future Research
Research about optimal diagnostic criteria are needed to identify PPI-resistant GERD patients that would benefit from switch to a potassium channel acid blocker, like vonoprazan. Additionally, research towards early identification of patients with functional heartburn or other symptoms of visceral hypersensitivity is key, as these patients would benefit from neuromodulators or behavioral intervention.
Conflict of Interest
Dr. Abu-Heija reports no potential conflicts of interest. Dr. Schoenfeld has been an advisory board member and speaker for Phathom Pharmaceuticals. Dr. Lynch has been an advisory board member and speaker for Medtronic and an advisory board member for Lucid Diagnostics, Sanofi, and Takeda Pharmaceuticals.
@renayadlapati
@jpandolfinomd
@KristleLynchMD
REFERENCES
- Katz PO, Dunbar KB, Schnoll-Sussman F, et al. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am J Gastroenterol 2022; 117: 27-56.
- Laine L, DeVault K, Katz P, et al. Vonoprazan versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology 2023; 164: 61-71.
- Katzka DA, Kahrilas PJ. PCAB Suppression of Gastric Acid in Erosive Esophagitis: Is Stronger and Longer Better? Gastroenterology 2023; 164: 14-15.
Download the article summary (PDF)

