In Case You Missed It
Test and Treat Helicobacter pylori in First-Degree Relatives of Gastric Cancer Patients-Reduces Their Risk of Gastric Cancer
Philip Schoenfeld, MD, MSEd, MSc (Epi)

Chief (Emeritus)-Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI
______________________________________________________________________________________________________
This article reviews Choi IJ, Kim CG, Lee JY, et al. Family History of Gastric Cancer and Helicobacter Pylori Treatment. N Engl J Med 2020; 382: 427-36. doi: 10.1056/NEJMoa1909666
Access this article through PubMed
Correspondence to Philip Schoenfeld, Editor-in-Chief. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Does Helicobacter pylori treatment reduce the risk of gastric cancer in individuals with a family history of gastric cancer?
Design:Single-center, randomized controlled trial.
Setting: National Cancer Center, South Korea.
Patients: Included patients were: (a) 40-65 years old; (b) first-degree relative with gastric cancer whose diagnosis was histologically confirmed by National Cancer Center; and (c) screening esophagogastroduodenoscopy (EGD) confirmed H. pylori infection by rapid urease test and wright-giemsa stain from gastric biopsies. Patients with a history of gastric cancer, peptic ulcer, and prior H. pylori eradication therapy were excluded.
Exposure/Intervention: Eligible patients were randomized to amoxicillin 1000 mg, clarithromycin 500mg, and lansoprazole 30 mg b.i.d. X 7 days vs placebo tablets. Patients then underwent surveillance endoscopy every 2 years with directed biopsies of suspicious lesions to identify gastric adenomas or carcinomas. At first surveillance endoscopy, H. pylori status was checked with rapid urease test, but patients and physicians were blinded to results and patients were not retreated if positive. At the final or close-out endoscopy, H. pylori status was again re-checked and salvage therapy provided if H. pylori infection was noted by rapid urease test.
Outcome: The primary endpoint was gastric cancer. Preferred secondary endpoints included development of gastric cancer based on H. pylori eradication status at surveillance EGD (accounting for patients with failed eradication treatment), gastric adenoma, and overall survival.
Data Analysis: Modified intention-to-treat analysis which excluded patients who never started H. pylori treatment/placebo or for whom no follow-up data was available. Kaplan-Meier analysis to assess primary and secondary endpoints. Log-rank test used to compare rates of gastric cancer. Cox proportional hazards used to estimate hazard ratios.
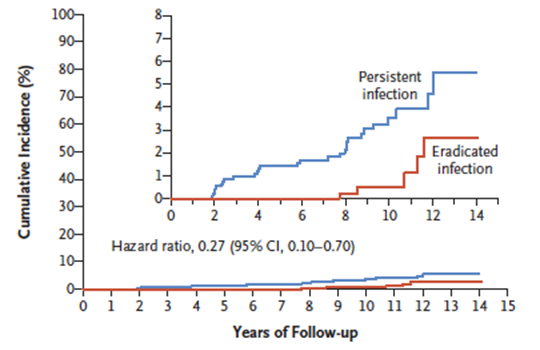
Results: Overall, 1,676 individuals were randomized (mean age: 49 years old; 49% male; 85%-86% with a single FDR with gastric cancer). Successful H. pylori eradication occurred in 70.1% of treated patients and 7.1% of placebo-treated patients at surveillance EGD. Median duration of follow-up for gastric cancer diagnosis was 9.2 years (IQR: 6.2-10.6; maximum 14.1). In the modified intention to treat analysis, placebo-treated patients were more likely to be diagnosed with gastric cancer vs antibiotic-treated patient: 2.7% vs 1.2%, P = 0.03; hazard ratio (HR)= 0.45; 95% confidence interval (CI): 0.21-0.94. Individuals with persistent H. pylori infection were significantly more likely to develop gastric cancer vs individuals with successful eradication: 2.9% vs 0.8%; HR = 0.27; 95% CI: 0.10-0.70 (Figure 1). There were no significant differences in placebo-treated patients vs antibiotic-treated patients for overall mortality (2.0% vs 1.7%) or gastric adenoma (1.5% vs 1.7%).
Funding: National Cancer Center, South Korea.
Figure 1. Cumulative Incidence of Gastric Cancer Based on H. pylori eradication status
COMMENTARY
Why Is This Important?
The development of gastric cancer is most likely due to a complex interaction of genetic predisposition, environmental factors, and H. pylori infection. Having first-degree relatives of gastric cancer patients is associated with a 2-3 fold increase in the risk of gastric cancer, and first-degree relatives of gastric cancer are more likely to have H. pylori infection compared to controls. Partly based on these observations, the Houston1 and Maastricht2 Consensus Conference reports on H. pylori management recommend that first-degree relatives of gastric cancer patients should be tested for H. pylori. However, the 2017 ACG Clinical Guideline on Treatment of H. pylori Infection3 is silent about testing and treating H. pylori in first-degree relatives of gastric cancer patients due to a paucity of evidence about its benefit. Given that gastric cancer is the one of the most common cancers worldwide, this question needs to be resolved, and the landmark RCT conducted by Choi and their colleagues at the Center for Gastric Cancer at the National Cancer Center in South Korea provides crucial data.
Key Study Findings
Successful eradication of H .pylori infection in first-degree relatives of gastric cancer patients decreased the risk of gastric cancer by more than 70%: HR= 0.27; 95% CI: 0.10-0.70.
Caution
This is another remarkably well-designed study. Since the authors used a placebo control, identified if patients had successful H. pylori eradication at
surveillance EGD, enrolled a large study population that was evaluated for an extended period (median follow-up of 9.2 years), and had virtually complete data collection, this study provides a precise estimate of gastric cancer reduction after successful H. pylori eradication in this high-risk population. As noted by the study investigators, the use of placebo in this trial could raise concerns, but H. pylori testing and treatment was not covered in asymptomatic individuals when this study was conducted in South Korea. Also, the generalizability of these data to non-East Asian populations may be limited since the development of gastric cancer is a complex multi-step process which is influenced by genetics and environmental factors as well as exposure to H. pylori.
The 7-day clarithromycin-based triple therapy was sub-optimal as evidenced by only achieving successful H. pylori eradiction in 70.1% of study patients randomized to treatment. Given the risk of clarithromycin resistance in the US, clarithromycin-based therapies should be used with caution, if at all. Bismuthbased quadruple therapy and rifabutin-based triple therapy for 14 days are preferred.4 Vonaprazam-based dual and triple therapies were also recently approved by the FDA.5 Vonaprazam, a potassium-competitive acid blocker, produces earlier and more potent acid suppression than conventional proton pump inhibitors. In clarithromycin-resistant H. pylori strains, vonaprazam dual therapy is much more effective vs standard 14-days of clarithromycin-based triple therapy (70% vs 32%).5
My Practice
Although there may be important genetic and environmental differences in US patients compared to East Asian patients, the potential benefits of testing and treating first-degree relatives of gastric cancer patients seems to far outweigh potential risks. Therefore, when I make a new diagnosis of gastric cancer in my Veteran population, I advise family members to get tested for H. pylori and treated if positive.
As noted above, my preferred therapy is bismuth-based quadruple therapy and I don’t recommend clarithromycin-based triple therapy routinely. In the near future, I may use vonaprazam dual therapy (20 mg vonaprazam b.i.d. plus 1000mg amoxicillin tid for 14 days) depending on cost and availability. Currently, I’m limited by my formulary to only using rifabutin-based triple therapy for salvage therapy. It’s also worth emphasizing the ACG Guideline recommendation that post-treatment testing should be performed routinely to ensure successful eradication. I prefer to do this with stool antigens for H. pylori and have the specimen collected at least 4 weeks after completing antibiotics and 2 weeks after discontinuing acid suppression therapy.
For Future Research
Prospective cohort data or retrospective case-control studies in US populations would be helpful. It would be unethical to perform a placebo-controlled trial in light of the data from Choi and colleagues.
Conflicts of Interest
Dr. Schoenfeld reports being an advisory board member and consultant for Phathom Pharmaceuticals.
References
1. El-Serag H, Kao JY, Kanwal F, et al. Houston Consensus Conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018; 16: 992-1002.
2. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht V/Florence Consensus Report. Gut 2017; 66: 6-30.
3. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017; 112: 212-38.
4. Howden C, Graham DY. Recent Developments Pertaining to H. pylori Infection. Am J Gastroenterol 2021; 116: 1-3.
5. Voquenza Prescribing Information. Phathom Pharmaceuticals. Accessed at www.accessdata.fda.gov on May 10, 2022.