In Case You Missed It
Low Grade Dysplasia in Barrett’s Esophagus–Get Two Expert GI Pathologist Reviews and Repeat EGD After PPI Treatment
 Shria Kumar, MD, MSCE1 and Gary W. Falk, MD, MS2
Shria Kumar, MD, MSCE1 and Gary W. Falk, MD, MS2
1Assistant Professor, Division of Digestive and Liver Diseases, University of Miami Miller School of Medicine, Miami, Florida
2Professor of Medicine, Division of Gastroenterology and Hepatology, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania
This article reviews: Vennalaganti P, Kanakadandi V, Goldblum JR, et al. Discordance Among Pathologists in the United States andEurope in Diagnosis of Low-Grade Dysplasia for Patients With Barrett’s Esophagus. Gastroenterology 2017 Feb;152(3):564-570.e4.
Correspondence to Shria Kumar, MD, MSCE, Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Question: How well do expert pathologists agree regarding the diagnosis of low grade dysplasia (LGD) in Barrett’s Esophagus(BE)?
Design: First, 3 US-based expert pathologists discussed the diagnostic criteria for LGD, to distinguish inflammatory predominant vs dysplasia predominant LGD. Then, 7 experienced pathologists (4 from the US, 3 from Europe) reviewed pathology slides of patients with BE with varying degrees of dysplasia in a random and blinded fashion.
Setting: Seventy-nine randomly selected pathology slides were obtained from the Veterans Affairs Medical Center in Kansas City and the Cleveland Clinic Foundation.
Samples: Slides included 23 non-dysplastic BE, 22 LGD, and 34 high-grade dysplasia (HGD). Slides were selected in a manner to represent those normally seen in tertiary care centers. There was no accompanying clinical information, and no areas were marked. Slides were processed by standard protocols, similar to standard clinical care.
Interventions: The pathologists received slides in a random fashion, and were aware they were participating in a research study regarding dysplasia. Each pathologist received a case report form to fill out per sample, which included the criteria for arriving at the diagnosis, the degree of
weighting placed on each of the criteria, and the diagnosis: either non-dysplastic, LGD-dysplasia predominant, LGD-inflammation predominant, or HGD. Pathologists were also asked to indicate if they had “high confidence” in their diagnosis.
Outcomes: Inter-observer agreement was the primary outcome for the study. A second analysis was conducted to evaluate which histologic features influenced the final diagnosis.
Data Analysis: Inter-observer agreement was calculated and reported using the kappa statistic, κ. Table 1 depicts how the kappa statistic is interpreted. The second analysis was conducted via a multinomial logistic regression analysis, using histologic features as covariates and the grade of dysplasia as the outcome variable, with non-dysplastic BE as the reference category.
Results: Inter-observer agreement is depicted in Figure 1. The overall kappa value was moderate at 0.43 (95% CI, 0.42-0.48). LGD had the lowest level of agreement: 0.11 (95% CI, 0.004-0.15), followed by non-dysplastic BE: 0.22 (95% CI, 0.11-0.29), then HGD: 0.43 (95% CI, 0.36-0.46).
High-confidence in diagnosis improved inter-observer agreement somewhat to 0.57 (95% CI, 0.45-0.62. Notably, high confidence in diagnosis among the 3 European pathologists led to considerably higher inter-observer agreement 0.80 (95% CI, 0.74-0.97) compared to the 4 US pathologists: 0.63 (95% CI, 0.61-0.66). When stratified by degree of dysplasia, interobserver agreement was consistently higher among the European than the American pathologists.
In the logistic regression, the diagnosis of LGD was mainly associated with the presence of cytologic atypia, nuclear hyperchromasia, and nuclear crowding. HGD was associated with glandular crowding, cytological atypia, nuclear enlargement, and irregular nuclear contours. US based pathologists diagnosed inflammation predominant LGD, dysplasia predominant LGD, and HGD based on the presence of a median of 5, 6, and 7 criteria, respectively. European pathologists diagnosed inflammation predominant LGD, dysplasia predominant LGD, and HGD based on the presence of a median of 3, 4, and 5
criteria, respectively.
Funding: None.
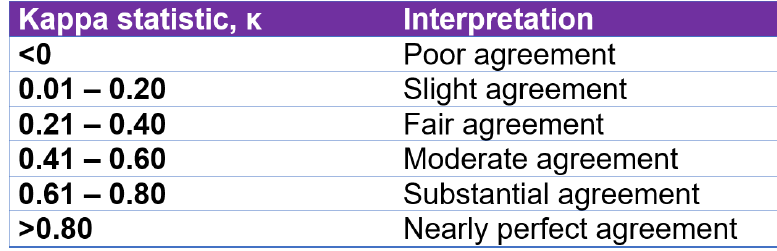
Table 1. How to interpret a Kappa statistic
__________________________________________________________________________________________

Figure 1. Inter-observer agreement between pathologists, by degree of dysplasia
__________________________________________________________________________________________
COMMENTARY
Why Is This Important?
Endoscopic therapies for BE with dysplasia are a mainstay of BE treatment. At the same time, endoscopic eradication therapies are not indicated for non-dysplastic BE, so getting the diagnosis of dysplasia right is of paramount importance for treatment decisions. As endoscopic eradication therapy typically involves multiple endoscopies for ablation or endoscopic mucosal resection, and given the costs and risks associated with these procedures, accurate diagnosis of dysplasia is essential. An accurate diagnosis of the degree of dysplasia is also important in counseling patients for their risk of progression to esophageal adenocarcinoma. Multiple studies have found widely disparate rates of progression from LGD to cancer,1,2 from <1% 3,4 to over 10% 5,6. These wide ranges of progression rates make it difficult to both understand the natural history of LGD and offer patients appropriate risk estimates of progression.
It has been noted in particular that Barrett’s esophagus patients in Europe with LGD have higher rates of progression than do patients in the US with LGD.7 One hypothesis for these widely disparate rates is that LGD is difficult to diagnose, and there is not always pathologist consensus, due to the subjective nature of some LGD criteria, and the overlap between LGD and Table 1. How to interpret a Kappa statistic inflammation and reactive changes. In fact, there is no clear gold standard for LGD, and there exist marked differences in LGD interpretation by pathologists in different geographic regions, and even academic vs. community based pathologists.8 There are also some data to suggest that there may be overdiagnosis of LGD in the US, particularly among pathologists who are less among pathologists who are less experienced in BE,9,10 and this overdiagnosis is accompanied a low risk of progression to HGD or esophageal adenocarcinoma. One study from the Netherlands demonstrated that expert review of histology specimens resulted in the downstaging of 73% of what were initially LGD specimens, to non-dysplastic BE.11 Recently, an updated ACG guideline on the Diagnosis and Management of Barrett’s Esophagus was published.12 It contains a strong recommendation to utilize endoscopic eradication therapy in patients with BE with HGD, but only a conditional recommendation for endoscopic eradication therapy in patients with BE with LGD. It further emphasizes that surveillance biopsy intervals are based on the degree of dysplasia. Importantly, although there is a low level of evidence in this arena, there is a strong recommendation to have dysplasia of any degree confirmed by a second pathologist with expertise in GI pathology. This underlines the notion that pathologic diagnosis is the cornerstone of esophageal adenocarcinoma prevention.
Key Study Findings
This is a well-designed study to evaluate the previously established observation that inter-observer agreement in LGD is poor, even among expert GI pathologists. The study demonstrates that LGD has the lowest inter-observer agreement for diagnosis, followed by non-dysplastic BE, and then HGD. Importantly, it demonstrates that European pathologists tend to have higher inter-observer agreement than their US counterparts. Lastly, it demonstrates that even with high confidence, inter-observer agreement regarding the degree of dysplasia is lacking.
Caution
Prior to having pathologists review slides, a consensus committee determined the criteria for LGD with predominantly inflammatory changes vs LGD with predominantly dysplastic changes. While inflammatory changes can muddle the diagnosis of LGD, it is not standard clinical practice to sub-divide LGD in this manner, and pathologists had to elect one subtype of LGD. This may have led to a greater decrease in inter-observer agreement than would be normally seen in clinical practice. The selected slides also introduced some bias, and there was a high representation of HGD in the slides (43%). In routine clinical practice, non-dysplastic BE is the most common finding. Lastly, the pathologists were all experienced pathologists and were aware they were participating in a research study. This reduces the generalizability of the study findings – but with such low inter-observer agreement among experienced GI pathologists, the inter-observer agreement may be even lower, particularly among those not at high-volume BE centers.
My Practice
This study supports our own practice at our respective academic centers, where we ensure at least 2 expert GI pathologists review dysplasia diagnoses, particularly for LGD. After confirmation, patients with BE and LGD diagnoses are counseled carefully, taking into consideration personal risk factors, comorbidities, age, and overall health status. Repeat endoscopy is performed after 3 months of proton pump therapy (Figure 2 depicts our approach to patients with LGD who present to our practice). For patients with confirmed LGD, we recommend endoscopic eradication therapy to reduce the risk of progression to HGD or esophageal cancer, but also believe that endoscopic surveillance is an acceptable alternative.12 Overall, this study further supports our understanding of the literature, and backs a salient hypothesis for the widely variable rates of progression to HGD or esophageal cancer in persons with LGD, and reaffirms that the diagnosis of LGD is complex, but of paramount importance.
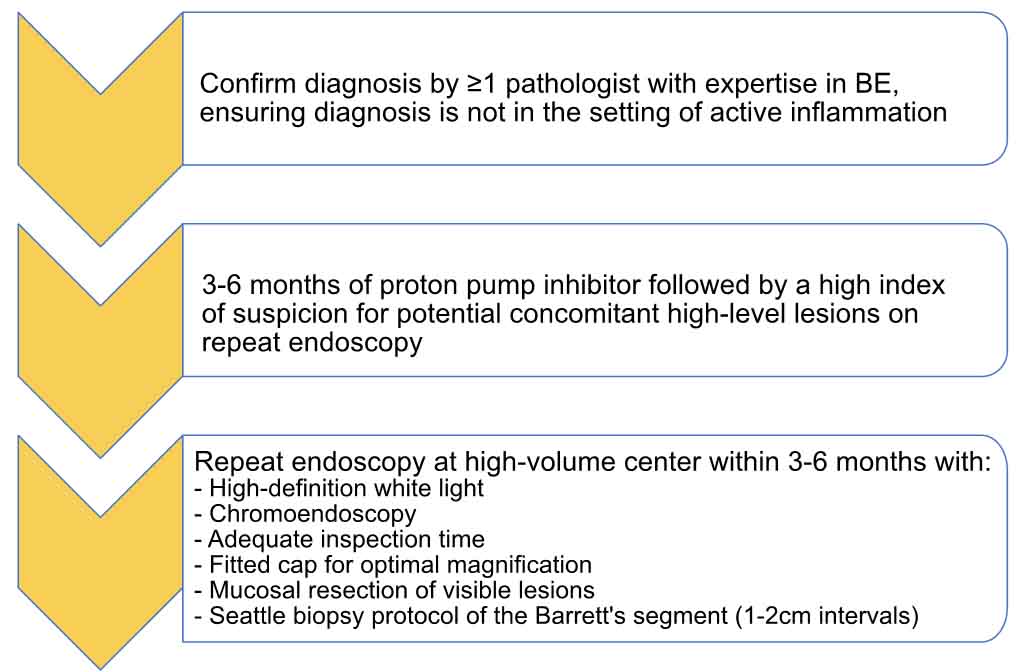
Figure 2. Our approach to a patient presenting with a diagnosis of Barrett’s esophagus with low-grade
dysplasia.
For Future Research
Newer methods of risk stratification for dysplasia management are on the horizon, and include assessment of p53 status,13,14 a tissue systems pathology test (TissueCypher)10, brushings with next generation sequencing (Barrett’s Aneuploidy Decision),15 and artificial intelligence.16 At this time, however, accurate pathologic diagnosis remains the best risk-stratification tool we have. Effectively counseling patients and providing them with information so they can partake in shared decision making that is in line with their values requires accurate diagnosis, risk estimates, and considerations of the risks and benefits of therapy. In addition, conjunction, endoscopic examination and biopsy sampling can lessen the burden on pathologists for the diagnosis of dysplasia in BE. Future research should focus on bolstering accurate diagnosis of LGD, interrogating the differences between European and US pathologist diagnostic methods, developing tools for pathologists and gastroenterologists to provide optimal risk stratification, and dysplasiaguided therapies to minimize their risk of future cancer. The SURVENT Trial is a multi-center trial that will address many of these questions, and we look forward to its findings (A Multicenter Randomized Controlled Trial of Surveillance versus. Endoscopic Therapy for Barretts Esophagus with Lowgrade Dysplasia, Project Number 1U34DK124174-01, www.clinicaltrials.gov).
Conflicts of Interest
G.W. Falk is a conclutant for Cernostics/Castle Biosciences, Lucid, Exact, Phathom, and CDX. S. Kumar has no conflicts of interest.
REFERENCES
- Codipilly DC, Chandar AK, Singh S, et al. The Effect of Endoscopic Surveillance in Patients With Barrett’s Esophagus: A Systematic Review and Meta-analysis.
Gastroenterology 2018;154(8):2068-2086 e5. - Krishnamoorthi R, Singh S, Ragunathan K, et al. Factors Associated With Progression of Barrett’s Esophagus: A Systematic Review and Meta-analysis. Clin Gastroenterol
Hepatol 2018;16(7):1046-1055 e8. - Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis.
Gastrointest Endosc 2014;79(6):897-909 e4; quiz 983 e1, 983 e3. - Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med 2011;365(15):1375-83.
- hoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311(12):1209-17.
- Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol 2010;105(7):1523-30.
- Falk GW. Current Management of Low-Grade Dysplasia in Barrett Esophagus. Gastroenterol Hepatol (N Y). 2017;13(4):221-225.
- Katzka DA, Falk GW. Management of Low-Grade Dysplasia in Barrett’s Esophagus: Incremental Progress Continues. Gastroenterology 2017;152(5):928-932.
- Falk GW. Low-grade dysplasia in Barrett’s esophagus: More than meets the eye? Gastrointest Endosc 2021;94(5):909-911.
- Iyer PG, Codipilly DC, Chandar AK, et al. Prediction of Progression in Barrett’s Esophagus Using a Tissue Systems Pathology Test: A Pooled Analysis of International
Multicenter Studies. Clin Gastroenterol Hepatol. Feb 22 2022. - Duits LC, Phoa KN, Curvers WL, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert
pathology panel. Gut. May 2015;64(5):700-6. - Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol 2022;117(4):559-587.
- Redston M, Noffsinger A, Kim A, et al. Abnormal TP53 Predicts Risk of Progression in Patients With Barrett’s Esophagus Regardless of a Diagnosis of Dysplasia. Gastroenterology 2022;162(2):468-481.
- Kastelein F, Biermann K, Steyerberg EW, et al. Aberrant p53 protein expression is associated with an increased risk of eoplastic progression in patients with Barrett’s
oesophagus. Gut 2013;62(12):1676-83. - Douville C, Moinova HR, Thota PN, et al. Massively Parallel Sequencing of Esophageal Brushings Enables an Aneuploidy-Based Classification of Patients With Barrett’s
Esophagus. Gastroenterology 2021;160(6):2043-2054 e2.
16. Hamade N, Sharma P. ‘Artificial intelligence in Barrett’s Esophagus’. Ther Adv Gastrointest Endosc 2021;14:26317745211049964.

