Post-Endoscopy Esophageal AdenoCarcinoma: Take a PEEC at Endoscopy Quality in Barrett’s Esophagus

Jennifer M. Kolb MD, MS
Assistant Professor of Medicine, Division of Gastroenterology, Hepatology, and Parenteral Nutrition, VA Greater Los Angeles Healthcare System, David Geffen School of Medicine at UCLA, Los Angeles, CA.
This summary reviews Wani S, Holmberg D, Santonin G, et al. Magnitude and time-trends of post-endoscopy esophageal adenocarcinoma and post-endoscopy esophageal neoplasia in a population-based cohort study: The Nordic Barrett’s Esophagus Study. Gastroenterology 2023; 165:909-19.
Access the article through PubMed
Correspondence to Jennifer M. Kolb, MD, MS. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Among a cohort of adults with newly diagnosed Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC), how many of these cancers are categorized as post-endoscopy esophageal adenocarcinoma (PEEC) and post-endoscopy high-grade dysplasia + EAC (PEEN)?
Design/Setting: The Nordic Barrett’s Esophagus Study (NordBEST) was a population-based cohort study using national patient registries from 3 Nordic countries: Denmark, Finland, and Sweden (Figure 1).
Patients: Adult patients aged >18 diagnosed with BE (with an accompanying endoscopy code) during the time period January 1, 2006, through December 31, 2020, were included in the cohort. Patients with prior esophageal or gastric surgery or cancer were excluded. Participants were followed until a diagnosis of high-grade dysplasia (HGD) or EAC, death, or the end of the study period.
Study Definitions: PEEC were defined as EAC, and PEEN was defined as EAC or HGD diagnosed between 30-365 days from the index endoscopy where BE was identified. The purpose of the 30-day time lag was to allow for additional procedures required to appropriately diagnose or stage any neoplasia identified on the index exam as well as completion of the pathology report. Cancers that were diagnosed between 0-29 days of the index endoscopy/BE diagnosis were categorized as index EAC and those diagnosed beyond 1 year as incident EAC.
Outcomes/Analysis: The primary outcome was the rates of PEEC and PEEN reported as incidence rates (IRs) per 100,000 person-years for the entire study period and for 3 calendar periods: 2006-2010, 2011-2015, and 2016-2020. Incidence rate ratios (IRRs) of EAC were computed using Poisson regression to compare the incidence of PEEC vs incident EAC. For the outcome of PEEN, only data from Sweden could be used since this was the only country that reported on HGD.
Various sensitivity analyses were conducted to evaluate the impact of modifying the 30-365 day time interval for occurrence of PEEC/PEEN to a) 30 days – 3 years and b) 6 months to 3 years. In analysis a, changing the upper limit of the time window to 3 years was done given that most endoscopists would recommend a surveillance interval of 3 years for non-dysplastic BE. The 6 months (analysis b) was chosen to allow for the possibility of erosive esophagitis on index endoscopy requiring follow up endoscopy to document healing and evaluate for BE.
Funding: Swedish Research Council (2019-00209)
Results: Between 2006-2020, there were 20,588 patients with newly diagnosed BE (14.8% Denmark, 20.5% Finland, 64.6% Sweden) and 293 cases of EAC (0.01%). Of these cases, 69 (23.5%) were categorized as PEEC, 14.7% as index EAC, and 61.8% as incident EAC. The IRs for PEEC was 392/100,000 person-years (95%CI 309-496) and lower for incident EAC 208 (95%CI 180-241). Among 279 patients diagnosed with HGD/EAC (Sweden only), 17.2% were categorized as PEEN with an IR of 421/100,000 person-years (95% CI 309-496).
Time trend analysis using 5-year intervals demonstrated rising incidence of PEEC/PEEN (p=0.02) with no change in IRs of incident EAC. Predictors of PEEC were older age and male sex. Sensitivity analyses using different definitions/time windows to categorize PEEC demonstrated largely similar results, or an even higher proportion of these cases, as well as a consistent increase in incidence across time-trend analyses.
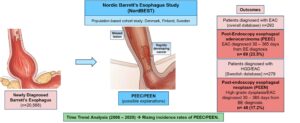
Figure 1. Study results and outcomes. Used with permission from Elsevier, copyright 2023.
COMMENTARY
Why Is This Important?
The majority of EAC cases present at a late stage associated with poor survival. Barrett’s esophagus (BE) is the known precursor lesion to EAC and provides the opportunity to intervene through screening in at risk individuals and enrolling those with BE in surveillance or treatment programs. The goal of surveillance is to identify low-grade or high-grade dysplasia or early cancer at a treatable stage. However, current early cancer detection practices are ineffective. One potential explanation for these limitations is PEEC/PEEN, similar to the concept of post-colonoscopy colorectal cancer and related to endoscopy quality. These cancers are found in patients with BE within a short time frame after a negative upper endoscopy and it is thought that they might have been missed on the initial examination, or perhaps have rapidly progressive biology.
The proportion of PEEC in EAC cases has been evaluated in several prior studies. An updated systematic review and meta-analysis of 52 studies with 145,726 patients demonstrated a PEEC rate of 21% (95% CI 13-31) and PEEN rate of 26% (95% CI 19-34) which were both lower among studies with only non-dysplastic BE (PEEC 17%, PEEN 14%).1 The proportion of PEEC increased over time from 5% in studies prior to 2000 to 30% in studies after 2015. Most of this US data comes from heterogenous observational studies in diverse practice settings that used different definitions of PEEC/PEEN and varying endoscopic techniques. The present study is unique and important because it is the first population-based report on the magnitude of PEEC/PEEN.
Key Study Findings
Caution
The study provided robust data from 3 countries on PEEC and is the only population-based report on this. However, the outcome of HGD was only reported in the Swedish database. There was no patient level, provider level, or center level data. It is still unclear why the rates of PEEC/PEEN are increasing despite stabilizing EAC incidence rates in these countries and endoscopist-level limitations in performance of EGD can’t be identified. The authors provide some hypotheses- poor quality endoscopy due to training issues or high pressure for clinical volume, significant use of endoscopy in the first year after a new BE diagnosis- but these require validation in other datasets.
My Practice
At the present time, it is believed that most cases of PEEC/PEEN can be attributed to missed lesions (versus aggressive biology). Accordingly, an international expert panel suggested multiple strategies to reduce the rates of PEEC/PEEN which all emphasize the importance of a high-quality endoscopic examination. I follow a 10-step approach (Table 1) to a high quality endoscopic exam in a patient with known or suspected BE.2-3 Some highlights of this exam include 1) use of standardized reporting systems to describe anatomic landmarks, the Barrett’s segment, and any visible lesions, 2)use of high definition white light endoscopy and chromoendoscopy, 3) spending adequate time inspecting the BE segment, 4) Seattle protocol sampling strategy (4 quadrant biopsies every 1-2cm along the BE segment) along with targeted biopsies, 5) adherence to guideline recommended surveillance intervals for NDBE.
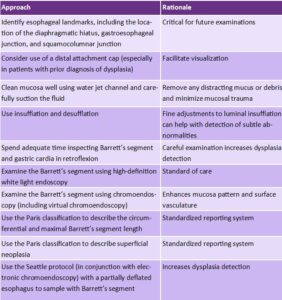
Table 1. Ten step approach to endoscopic examination of Barrett’s esophagus.2
For Future Research
More data using a large, nationally representative sample is needed to describe the rates of PEEC/PEEN in the US population. Future studies should focus on the clinical and endoscopic characteristics of PEEC and try to elicit the contributing factors for these cases. Additionally, efforts to standardize how PEEC/PEEN rates are calculated in an automatic fashion can inform mechanisms for its use as a quality indicator with defined performance thresholds.
Conflict of Interest
Dr. Kolb reports no potential conflict of interest.
@LagergrenJesper
REFERENCES
- Sawas T, Majzoub A, Haddad J, et al. Magnitude and time-trend analysis of post-endoscopy esophageal adenocarcinoma: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022; 20: e31-e50..
- Kolb JM, Wani S. Barrett’s esophagus: current standards in advanced imaging. Transl Gastroenterol Hepatol 2021;6:14.
- Shaheen N, Falk G, Iyer P, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol 2021; 117: 559-87.

