Disposable Elevator Caps for Duodenoscopes Decrease Contamination Without Hindering Technical ERCP Performance: The ICECAP Trial
 Shria Kumar, MD, MSCE
Shria Kumar, MD, MSCE
Assistant Professor, Division of Digestive and Liver Diseases, University of Miami Miller School of Medicine, Miami, FL
This article reviews Forbes N, Elmunzer BJ, Allain T, et al. Effect of Disposable Elevator Cap Duodenoscopes on Persistent Microbial Contamination and Technical Performance of Endoscopic Retrograde Cholangiopancreatography: The ICECAP Randomized Clinical Trial. JAMA Intern Med 2023;183(3):191-200. doi: 10.1001/jamainternmed.2022.6394.
Access the article through PubMed
Correspondence to Shria Kumar, MD, MSCE. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Do duodenoscopes with disposable elevator caps decrease persistent microbial contamination compared to standard design scopes without impacting technical performance in endoscopic retrograde cholangiopancreatography (ERCP)?
Design: Parallel-arm, multi-center randomized clinical trial (RCT). Immediately preceding ERCP, patients were randomly assigned in a 1:1 ratio to undergo ERCP using a disposable elevator cap duodenoscope (ED34-i10T2, Pentax Medical) or a standard duodenoscope (ED34-i10T, Pentax Medical)
Setting: Two tertiary-care ERCP centers in Canada, between December 1, 2019 and February 28, 2022, including a pause due to the COVID pandemic from March 2020 to September 2020.
Patients: Five hundred eighteen patients aged 18+ years who were undergoing ERCP for any indication were included. Exclusion criteria included inability/unwillingness to provide informed consent, pregnancy, breastfeeding, or potential inability to complete a 30-day follow-up.
Intervention: The use of duodenoscopes with disposable elevator caps was compared with duodenoscopes with a standard design.
Outcomes: Co-primary outcomes were 1) persistent microbial contamination of the duodenoscope elevator or channel (superiority outcome), and 2) technical success of ERCP according to a priori criteria (noninferiority outcome with an a priori noninferiority margin of 7%). Persistent microbial contamination was defined as either growth of 10 or more colony-forming units (CFUs) of any organism or any growth of gram-negative bacteria, within 72 hours of plating. Technical success of ERCP was determined independently by 2 persons blinded to group assignment based on a priori definitions and focused on successful completion of procedure according to indication (e.g., removal of stones in cases done for choledocholithasis, stent placement across stricture for a biliary stricture, or cholangioscopy completion in cases where visualization was planned).
Secondary outcomes included mortality, patient tolerability, and adverse events within 30 days of ERCP (cholangitis, pancreatitis, bleeding, perforation, and cardiopulmonary events).
The duodenoscopes in both study arms were required to have been in clinical use between 12 months and 24 months. Prior to sample collection to assess for the primary outcome, the duodenoscopes underwent 2 cycles of high-level disinfection followed by steam sterilization. Following this, they underwent point-of-care adenosine triphosphate (ATP) scanning, with any failed scan resulting in the scope being sent for another disinfection cycle. ATP scanning looks for bioluminescence from microbial residue. Once they have passed ATP scanning, they were “deemed cleared for clinical use,” and microbiological sampling was performed within 60 minutes. Two samples were acquired from each duodenoscope: 1 from the elevator area (the elevator itself for standard duodenoscopes, and the cap attachment point for disposable elevator cap duodenoscopes) and 1 from the instrument channel.
Statistical Analysis: Intention-to-treat analysis without adjustment using chi-square tests.
Funding: Research support was provided by the ASGE and the Canadian Institutes of Health Research. Pentax Medical provided unrestricted temporary use of duodenoscopes. None of the parties were involved in study conception, design, or execution, or in the interpretation and/or reporting of results.
Results: There were 518 patients enrolled and split evenly between disposable elevator cap group (n=259) and standard duodenoscope group (n=259). Patient demographics included mean age of 60-61 years; indication for ERCP: suspected/confirmed biliary stone (38%-44%), suspected/confirmed biliary stricture (9%-12%), repeat ERCP including stent removal or exchange (20%-22%); and, American Society for Gastrointestinal Endoscopy (ASGE) Grade Procedural Complexity: Grade I/II (76%).
Based on a priori sample size calculations, 208 patients in the disposable elevator cap group and 214 patients in the standard duodenoscope group had their duodenoscopes sampled after high-level disinfection (microbiology outcome). All patients were included for the technical success outcome.
Persistent microbial contamination was detected in 11.2% of duodenoscopes in the standard duodenoscope arm and 3.8% of duodenoscopes in the disposable elevator cap duodenoscope arm (P = .004), corresponding to a relative risk (RR) of 0.34 (95% confidence interval [CI], 0.16-0.75) and number needed to treat of 13.6 (95% CI, 8.1-42.7) to avoid 1 persistent microbial contamination event. Persistent microbial contamination occurred most frequently in the instrument channel sample (Table 1).
Technical success with disposable elevator cap duodenoscopes was noninferior to that with standard duodenoscopes (94.6% vs 90.7%, P=0.13). There were no differences in mortality, patient tolerability, and adverse events.
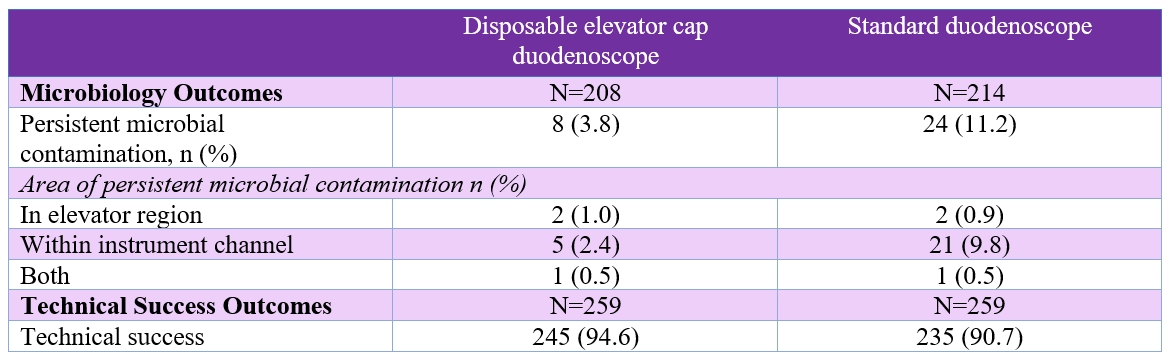
Table 1. Outcomes
COMMENTARY
Why Is This Important?
In 2013, the Centers for Disease Control and Prevention (CDC) alerted the Federal Drug Administration (FDA) to a potential association between multi-drug resistant bacteria and duodenoscopes. Upon further investigation, it became clear that these cases of infection were occurring despite confirmation that the users were following proper manufacturer cleaning and disinfection or sterilization instructions. The underlying theory suggests that if a person who is colonized undergoes ERCP, the duodenoscope can be colonized. If the colonization persists (even after cleaning), this can lead to transfer to the next person who undergoes ERCP with the duodenoscope, and possibly clinical infection.
Duodenoscopes are complex instruments. They include a working channel, through which instruments are passed, an elevator mechanism that allows for manipulation of devices through the papilla, and an O-ring that seals off the elevator channel from contamination. Contamination is possible either due to insufficient cleaning and reprocessing (due to the complex design), the development of a biofilm, and/or breaches of the O-ring seal.1
To address this issue, disposable elevator cap duodenoscopes and completely disposable duodenoscopes have both been introduced. Disposable duodenoscopes offer an attractive solution in theory, but they are expensive (particularly considering their single-used design), are technically inferior, and create medical waste.2 Disposable elevator cap duodenoscopes can theoretically address the concerns of infections while overcoming the limitations of disposable duodenoscopes.
Duodenoscope-related infections are rare, occurring in 0.01% of persons.3 While this may seem small, this estimate is from a systematic literature search of duodenoscope-related infections in the Netherlands, and was an important update in prior data that suggested the risk of duodenoscope-related infections was almost negligible. Furthermore, given that US endoscopists performed over 175,000 ERCPs in 2019, it is an important consideration that has been relatively under-investigated.4
Therefore, we commend the investigators for performing a very well-designed study to investigate tools to further minimize persistent microbial contamination of duodenoscopes after appropriate cleaning and disinfection.
In this RCT of 518 patients undergoing ERCP, duodenoscopes with disposable caps reduced persistent microbial contamination (RR, 0.34), with no differences in performance (technical success, 94.6% vs 90.7%) and safety outcomes. The most frequent area of persistent microbial contamination was within the instrument channel (as compared to the elevator area).
Caution
Duodenoscope-related infections are difficult to study. As the authors appropriately acknowledge, persistent microbial contamination is a surrogate outcome, which has limited correlation to clinically relevant duodenoscope-related infections that are much less common. This is not a criticism of the authors or study design, but simply reflects that it would be impractical to enroll the hundreds of thousands of patients needed to demonstrate a difference in duodenoscope-related infections.
My Practice
This is an evolving area that is growing in importance. Completely disposable duodenoscopes are not a practical solution currently because of high cost and limitations in technical performance. We are currently using disposable caps at one of our hospitals, and will begin using disposable caps at our hospital in the next few months.
For Future Research
Further validation of disposable caps should be performed across different settings (geographically and with different endoscope manufacturers). We need better surveillance protocols to identify, quarantine, and disinfect contaminated duodenoscopes since the accuracy of ATP scanning to look for bioluminescence from microbial residue misses contamination. Novel duodenoscope designs that make cleaning easier without sacrificing technical aspects are also needed.
Conflicts of Interest
Dr. Kumar reports no conflicts of interest.
@nauzerforbes (Nauzer Forbes)
@jelmunzer (B. Joseph Elmunzer)
REFERENCES
- Larsen S, Russell RV, Ockert LK, et al. Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. EClinicalMedicine 2020; 25: 100451.
- Bang JY, Sutton B, Hawes R, Varadarajulu S. Concept of disposable duodenoscope: at what cost? Gut 2019;68(11):1915-1917.
- Kwakman JA, Erler NS, Vos MC, Bruno MJ. Risk evaluation of duodenoscope-associated infections in the Netherlands calls for a heightened awareness of device-related infections: a systematic review. Endoscopy 2022;54(2):148-155.
- Peery AF, Crockett SD, Murphy CC, et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology 2022;162(2):621-644.

