Surveillance of Branch Duct IPMN – Enough is Enough, At Least in Older Adults and Small, Stable Lesions

Shria Kumar, MD, MSCE
Assistant Professor, Division of Digestive and Liver Diseases, University of Miami Miller School of Medicine, Miami, FL
This article reviews Marchegiani G, Pollini T, Burelli A, et al. Surveillance for Presumed BD-IPMN of the Pancreas: Stability, Size, and Age Identify Targets for Discontinuation. Gastroenterology. 2023 Oct;165(4):1016-1024.e5.
Access the article through PubMed
Correspondence to Shria Kumar, MD, MSCE. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: What patient groups with presumed branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) are at very low risk of malignant progression (where their likelihood of pancreatic cancer is no different from that of an age-matched general population)?
Design: Retrospective analysis of prospective collected data.
Setting: This was an international multicenter study, including centers in Europe, the United States, and Asia under the auspices of the Verona Evidence-Based Meeting on IPMN Consortium. Each institution prospectively collected data that included clinicopathologic data, including demographics, radiological and endoscopic characteristics of the cyst, surgical data, clinical data with comorbidities. IPMN-related features included CA19-9, cyst size, location, cyst wall, mural nodules, solid components, septae, and main pancreatic duct size. From the data, authors evaluated presumed BD-IPMN without worrisome features (WFs) or high-risk stigmata (HRS) at diagnosis who underwent surveillance. Table 1 lists WFs and HRS that may indicate need for more intense surveillance, interventional EUS, or surgery.
Patients: There were 3,844 adults with BD-IPMN, lacking any WF or HRS who were enrolled in surveillance programs. Median age was 66, and 60% were female. Initial BD-IPMN diameter was median 12mm (interquartile range [IQR] 9mm). BD-IPMN was a presumptive diagnosis based on the presence of 1 or more dilated branch ducts communicating with a nondilated main pancreatic duct (MPD) (5 mm or smaller) on high-resolution cross-sectional imaging or endoscopic ultrasound. Exclusion criteria included those who underwent surgery within 12 months of cyst detection, those with a prior history of pancreatic cancer or prior pancreatic surgery, and those with cysts suspicious for a diagnosis other than BD-IPMN.
After determining inclusion, clusters of individuals at risk for cancer development were defined according to cyst size and stability for at least 5 years, and age-matched controls were used for comparison using standardized incidence ratios (SIRs) for pancreatic cancer. The authors identified persons who had BD-IPMN that did not develop WF or HRS over 5 years (termed “Trivial BD-IPMN”).
Outcomes: The primary endpoint was the development of pancreatic cancer, defined as either IPMN with associated invasive carcinoma or IPMN with concomitant pancreatic ductal adenocarcinoma (PDAC). Secondary endpoints were development of WFs and HRS during follow-up, along with risk factors for developing pancreatic cancer, like cyst size, growth rate, and survival.
Statistical Analysis: Data were analyzed using Cox proportional hazard models to assess the association between WF/HRS development and overall survival. To calculate the SIR of pancreatic cancer of the cohort compared to the general population, the authors obtained sex-specific pancreatic cancer rates from the World Heath Organization’s International Agency for Research on Cancer (IARC). Age-standardized incidence was assessed.
Funding: This study was supported by funding from the Italian Ministry of Health (Grant FIMP-CUP Q8 1142 J38D19000690001).
Results: Of 3,844 patients with presumed BD-IPMN, 775 (20.2%) developed WFs and 68 (1.8%) HRS after a median surveillance of 4.4 years. Another 164 (4.3%) underwent surgery. For the entire study cohort, 1,617 (42%) remained stable without developing WFs or HRS for at least 5 years with another 1,220 (31.4%) remaining stable during less than 5 years surveillance.
Of the 775 who developed WF, 121 (15.6%) developed at least 1 other WF. Developing 2 of more WFs was associated with worse survival: hazard ratio (HR) 2.38 (95% confidence interval [CI] 1.47–3.86; P< 0.001) compared with the development of only 1 WF: HR 1.43 (95% CI 1.02–2.02; P=0.036). Developing HRS during surveillance was associated with the diagnosis of an invasive cancer at final pathological examination (26.9% vs 10.1%, P=0.042), whereas the development of a WF was not (P >0.05). No individual WF or HRS was associated the diagnosis of HGD, but an abrupt change in MPD caliber (P=0.021), a Ca19-9 ≥ 37 U/L (P=0.001) and the presence of jaundice (P=0.021) were associated with the diagnosis of an invasive cancer.
In patients with a Trivial BD-IPMN, the development of a WFs and/or HRS after the first 5 years of surveillance was associated with worse overall survival: WF : HR 2.79 (95% CI 1.46–5.32; P=0.002); HRS: HR 5.52 (95% CI 1.94–15.69; P=0.001).
SIR of developing pancreatic cancer
In patients 75+ years of age, the SIR of developing pancreatic cancer was 1.12 (95% CI 0.23-3.39), and in patients 65+ years with stable lesions smaller than 15 mm in diameter after 5 years, the SIR was 0.95 (95% CI 0.11-3.42). The disease-specific mortality for patients who did not develop WFs or HRS for at least 5 years was 0.3% (n = 5). Table 2 indicates SIR by subgroup.
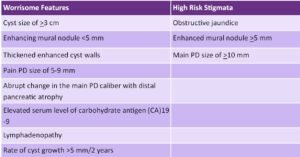
Table 1: Worrisome features and high-risk stigmata of IPMNs.
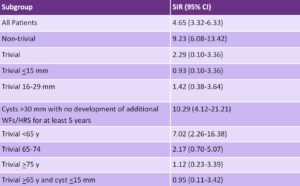
Table 2: Standardized incidence ratio (SIR) by branch-duct intraductal papillary mucinous neoplasms subgroup.

Figure 1. When to consider discontinuing surveillance if no worrisome features or high-risk stigmata.
COMMENTARY
Why Is This Important?
Incidentally detected pancreatic cysts are a burgeoning issue. With more frequent and higher quality cross-sectional imaging, the detection of pancreatic cysts has increased substantially over the last 2 decades – they are identified in at least ~10% of MRIs.1,2 It is thought that some of these may harbor malignant potential – though it is not as clear-cut a transition as the adenoma to carcinoma pathway seen in colorectal cancer. But as pancreatic ductal adenocarcinoma (PDAC) remains among the deadliest cancers – with a has a 5-year survival of less than 10% – and a rising incidence (increasing by 0.5% to 1.0% per year), it is a worrying issue to clinicians and patients alike.3,4
In response, guidelines for surveillance were formed. Multiple guidelines exist, including the ACG’s 2018 Clinical Guideline on the diagnosis and management of pancreatic Cysts.5 BD-IPMNs represent one of the more common types of pancreatic cystic lesions, and their surveillance entails cross-sectional imaging and/or endoscopic ultrasound. Surveillance can be offered until a patient is no longer a surgical candidate, though apart from suggestions of considering lengthening intervals if lesions are stable, there is little guidance regarding when to stop surveillance. Given the healthcare burden and cost associated with surveillance, as well as the impact on patients, studies evaluating whether and in whom surveillance can be stopped are critical.
Key Study Findings
Caution
The authors did an excellent job answering an important question in a clinically relevant way, but it is important to consider that the cohort consists of persons enrolled in surveillance programs at high-volume centers, portending bias. BD-IPMN was a presumptive (not confirmed) diagnosis, though that is reflective of real-world practice. The data was collected over 30 years, and there are variations in imaging (and particularly endoscopic ultrasound) that may introduce biases. And while surveillance was relatively short (median follow up just under 5 years), there were persons who developed WF or HRS after having stable findings for many years. Finally, while a multi-center international study, it would be important to know whether there are practice or patient differences across locations.
My Practice
My practice in this area mirrors what the authors advocate. I recommend stopping surveillance in individuals greater than 75 years of age with small and stable lesions. I liken this to colon cancer screening cessation, particularly in persons with comorbidities or marked frailty. For persons above 65 years of age, my approach is more individualized – but the present study encourages me to consider cessation, or at least a marked lengthening of surveillance interval for persons above 65 with a lesion that is ≤15mm. In general, I find that shared-decision making is best, particularly given the concern that pancreatic pathology can invoke in patients.
For Future Research
Ideally in the future, we will have guidance and recommendations for other patients or cyst characteristics for which we can stop or lengthen surveillance. We should also have (with ongoing studies such as CAPS and PRECEDE) a more concrete understanding of the potential “benefits” and “harms” of screening/surveillance – essential to shared-decision making. The authors’ study also highlights the need for diagnostic criteria and tools more nuanced and predictive than WF alone. Finally, we need further research on the carcinogenic pathway in pancreatic cancer, so we can better counsel patients on their risk.
Conflicts of Interest
Dr. Kumar reports no conflicts of interest.
REFERENCES
- de Oliveira PB, Puchnick A, Szejnfeld J, Goldman SM. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One. 2015;10(3):e0121317.
- Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. Sep 2010;105(9):2079-84.
- Kwakman JA, Erler NS, Vos MC, Bruno MJ. Risk evaluation of duodenoscope-associated infections in the Netherlands calls for a heightened awareness of device-related infections: a systematic review. Endoscopy. 2022; 54(2):148-155.
- Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: Update 2021. Gastroenterology. 2022;162(2):621-644.
- Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG clinical guideline: Diagnosis and management of pancreatic cysts. Am J Gastroenterol 2018; 113(4):464-479.

