Post-Colonoscopy Colorectal Cancer Due to Missed Polyps in Proximal Colon or Rectum with Sub-Optimal Bowel Cleansing
 Jeffrey Lee, MD, MPH
Jeffrey Lee, MD, MPH
Research Scientist and Attending Gastroenterologist, Kaiser Permanente San Francisco Medical Center, San Francisco, CA
This summary reviews Troelsen FS, Sørensen HT, Pedersen L, et al. Root-cause analysis of 762 Danish post-colonoscopy colorectal cancer patients. Clin Gastroenterol Hepatol 2023;21(12):3160-3169.e5.
Access the article through PubMed
Correspondence to Jeffrey Lee, MD, MPH. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: What are the causes of post-colonoscopy colorectal cancer (PCCRC), defined as first-time colorectal cancer (CRC) diagnosis > 6 months to 48 months after a negative colonoscopy (i.e., no evidence of CRC on index colonoscopy)?
Design: Retrospective cross-sectional study.
Setting: Central Denmark Region, which covers approximately 1.3 million individuals.
Patients: Using the Danish Cancer Registry and the Danish National Patient Registry from 1995-2021, 762 individuals with PCCRCs were identified. Among these PCCRC cases, 46.5% were females, 4.1% had a family hereditary colorectal cancer syndrome, 52.6% had a prior polypectomy, and 2.5% had an inflammatory bowel disease diagnosis.
Exposure: For each PCCRC case, manual chart review was performed to extract detailed information from colonoscopy reports, including colonoscopy indication, quality of bowel preparation, cecal intubation, colonoscopy findings and pathology reports. The most plausible cause of the PCCRC was then determined by performing a root-cause analysis using the new World Endoscopy Organization (WEO) consensus recommendations.
Outcome: Each PCCRC case were categorized as: a) possible missed lesion, prior examination adequate (i.e., cecum was reached, and the bowel preparation was adequate); (b) possible missed lesion, prior examination inadequate; (c) detected lesion, not resected; or (d) likely incomplete resection of previously identified lesion.
Data Analysis: Indication for colonoscopy was only provided on colonoscopy reports from 2014-2021, so analyses assumed that all colonoscopies performed from 1995-2013 were performed on symptomatic patients. Also, an additional 175 PCCRC cases were eliminated from analysis because insufficient data was available for root-cause analysis.
Funding: Danish Cancer Association and Novo Nordisk Foundation.
Results: Of the 762 PCCRCs, 15.2% of PCCRCs were located in the cecum, 15.7% in the ascending colon, and 23% in the rectum. The most plausible explanation for these PCCRCs were: Category A: 80.8% (possible missed lesion with a prior adequate examination); Category B: 4.7% (possible missed lesion with a prior inadequate examination); Category C: 3.4% (detected lesion but not resected); and, Category D: 11% (likely incomplete resection of previously identified lesion) (Figure 1).
Bowel preparations were recorded as: poor, fair, good, or excellent. For root-cause analysis, “fair” prep was considered adequate. When “fair” bowel preparation was re-classified as “inadequate,” then Category A (possible missed lesion-adequate exam) decreased from 80.8% to 63.4% and Category B (possible missed lesion-inadequate exam) rose from 4.7% to 22.2%.
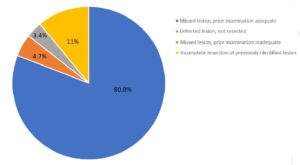
Figure 1. Plausible explanations of 762 post-colonoscopy colorectal cancer in Central Denmark.
COMMENTARY
Why Is This Important?
Multiple studies have shown that colonoscopy reduces CRC incidence and mortality.1 In the United States, colonoscopy is the most common screening test for CRC and is the primary diagnostic procedure for follow up after a positive fecal-based screening test and for evaluating signs and symptoms related to CRC. Unfortunately, colonoscopy is not perfect, and cancers can be diagnosed after a negative colonoscopy which did not detect cancer, or PCCRC.2,3 Recently, the WEO developed a consensus statement and methodology to better classify PCCRCs into their most plausible explanations.4 By understanding the root cause of PCCRC, endoscopists may better improve colonoscopy quality.
Only a few studies have utilized this methodology, and they demonstrated that most PCCRCs are likely due to missed lesions in the proximal colon. However, these studies were limited by their relatively small sample size.5,6 To address these limitations, the authors performed a root cause analysis for 762 PCCRC cases diagnosed in Central Denmark.
Key Study Findings
Caution
The WEO methodology for classifying PCCRC etiology does not adequately describe some situations where the root cause of the PCCRC is due to patient- or system-related failures (e.g., failure to schedule a surveillance colonoscopy). Insufficient data was available for multiple colonoscopies. Also, there was no reporting of adherence to quality standards for most colonoscopies (e.g., withdrawal time, performance of second view of rectum in retroflex view).
My Practice
As seen in this study and other prior studies,5,6 missed lesion is the most common explanation for PCCRCs diagnosed within 48 months after a clearing colonoscopy. This finding highlights the need for careful inspection of the colon during withdrawal, particularly in the right colon and rectum.
There are several tools and techniques that I use to optimize pre-cancerous lesion detection during withdrawal. First, it is critical to use a high definition colonoscope with image enhancement (e.g., narrow band imaging) capabilities to help detect and evaluate subtle lesions. Second, it is important to have a mindset for detecting flat polyps since these lesions are often missed. Third, I maximize mucosal exposure by “working the folds” (i.e., deflecting the tip of the colonoscope into the inner-haustral valley and exposing the proximal sides of each haustral folds), cleaning and suctioning any stool debris, and distending the lumen adequately. Fourth, I perform 2 or 3 passes in the right colon and rectum since adenomas, especially flat lesions, are often missed in this location. Lastly, when available, I often use a distal attachment device such as a clear translucent cap to help expose the proximal sides of each haustral fold and improve mucosal exposure.
In addition to missed lesions, incomplete resection is a critical modifiable factor for PCCRC that deserves more attention. There are several tips and techniques I like to share with my colleagues and fellows to reduce the chances of adenoma recurrence. First, give yourself time–never tackle a polyp you cannot finish during your assigned time slot. Second, be humble and refer any complex polyp to a colleague or referral center that specializes in advanced tissue resection. Third, always aim for en bloc resection using conventional or underwater EMR or ESD technique. Fourth, if en bloc is not feasible, make sure to take wide margins and ablate the edges of the defect with soft tip coagulation after piecemeal EMR. Fifth, carefully inspect the piecemeal EMR defect and remove any residual or visible islands using hot forceps avulsion. Lastly, emphasize to your patients that it is critical to come back for your surveillance colonoscopy in 6 months following your EMR or ESD.
For Future Research
Additional studies evaluating the root cause of PCCRC are needed, particularly PCCRC cases diagnosed after 4 years following a clearing colonoscopy. Studies about successful implementation of quality improvement of colonoscopy procedures with an emphasis on improving adenoma detection and performing high-quality resection of pre-cancerous lesions would also be useful.
Conflict of Interest
None to report.
REFERENCES
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315(23):2564-2575.
- Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370(14):1298-1306.
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362(19):1795-1803.
- Rutter MD, Beintaris I, Valori R, et al. World Endoscopy Organization consensus statements on post-colonoscopy and post-imaging colorectal cancer. Gastroenterology 2018 Sep;155(3):909-925.e3.
- Leung LJ, Lee JK, Merchant SA, et al. Post-Colonoscopy Colorectal Cancer Etiologies in a Large Integrated US Health Care Setting. Gastroenterology 2023;164(3):470-472.e3.
- Anderson R, Burr NE, Valori R. Causes of Post-Colonoscopy Colorectal Cancers Based on World Endoscopy Organization System of Analysis. Gastroenterology 2020 Apr;158(5):1287-1299.e2.

