What are the Causes of Post-Colonoscopy Colorectal Cancer?
Jeffrey Lee, MD, MPH
Research Scientist and Attending Gastroenterologist, Kaiser Permanente San Francisco Medical Center, San Francisco, CA
This summary reviews Leung LJ, Lee JK, Merchant SA, Jensen CD, Alam A, Corley DA. Post-Colonoscopy Colorectal Cancer Etiologies in a Large Integrated US Health Care Setting. Gastroenterology 2023;164(3):470-72.
Correspondence to Jeffrey Lee, MD, MPH. Associate Editor. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: What are the causes of post-colonoscopy colorectal cancer (PCCRC)?
Design: Retrospective cross-sectional study.
Setting: Community-based integrated healthcare setting in the United States (Kaiser Permanente Northern California).
Patients: A random sample of 533 PCCRCs were identified from January 1, 2006 to December 31, 2018. Among these PCCRC cases, 46.1% were female, 70% were non-Hispanic White, 7.1% had a family history of colorectal cancer (CRC) in a first-degree relative, 54.1% had diverticular disease, 41.5% had a prior adenoma diagnosis, 12.4% had a prior CRC diagnosis, and 7.8% had inflammatory bowel disease diagnosis.
Interventions/Exposure: For each PCCRC case, defined as a CRC occurring >6 months to 10 years after a negative colonoscopy (i.e., no evidence of CRC on examination), manual chart review was performed to determine the most plausible cause of the PCCRC using the World Endoscopy Organization (WEO) consensus recommendations.
Outcome: Each PCCRC case was categorized as the following: 1) likely new cancer; 2) possible missed lesion, prior examination adequate (i.e., cecum was reached, and the bowel preparation was adequate); 3) possible missed lesion, prior examination inadequate; 4) detected lesion, not resected; or 5) likely incomplete resection of previously identified lesion.
Results: Of the 533 PCCRCs, 197 (37.0%) were likely new cancers, which were diagnosed more than 4 years after a negative colonoscopy. For the remaining 336 PCCRCs diagnosed within 4 years of the negative colonoscopy, the most plausible explanation for these PCCRCs were as follows: 70.2% (236 of 336) were classified as possible missed lesion with adequate prior examination; 15.5% (52 of 336) were classified as possible missed lesion but the prior examination was inadequate; 11% (37 of 336) were classified as likely incomplete resection of a previously identified lesion; and 3.3% (11 of 336) were classified as detected lesion that was not resected (Figure 1).
Funding: This study was funded by the National Cancer Institute/National Institutes of Health.
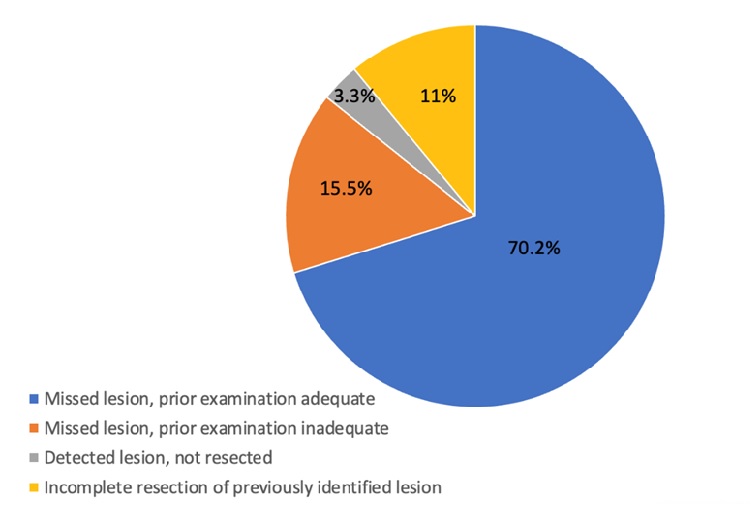
Figure 1. Causes of PCCRC within 4 years of negative colonoscopy (n=336).
COMMENTARY
Why Is This Important?
Multiple studies have shown that colonoscopy reduces CRC incidence and mortality.1,2 In the US, colonoscopy is the most common screening test for CRC and is the primary diagnostic procedure for follow-up after a positive fecal-based screening test and for evaluating signs and symptoms related to CRC.1,2 Unfortunately, colonoscopy is not perfect, and cancers can be diagnosed after a negative colonoscopy, which did not detect cancer—termed PCCRC.3 Although several studies have identified risk factors for PCCRC,4-7 few have provided the detail required to understand exactly what led to the PCCRC.3 Recently, the WEO developed a consensus statement and methodology to better classify PCCRCs into their most plausible explanations.3 However, only a few studies have utilized this methodology, which were all from Europe with small sample sizes (~40-100 PCCRC cases).8-10 To address these limitations, the authors performed a root cause analysis for 516 PCCRC cases diagnosed within a large and diverse community-based population in the United States using the WEO consensus recommendations and methodology.
Key Study Findings
Among the 533 PCCRC cases, nearly 40% were classified as new cancers per the WEO methodology because they were diagnosed >4 years after the negative colonoscopy. The remaining cases (62.2%) were diagnosed within 48 months of a negative colonoscopy that did not identify a cancer; of these, 72.6% were classified as possible missed lesion with a prior adequate examination; 12.5% were classified as possible missed lesion but prior examination was inadequate; 11.5% were due to incomplete resection of a previously identified lesion; and 3.4% were due to a detected lesion that was not resected (Figure 1).
This supports targets for reducing the frequency of PCCRCs, including increasing adenoma detection rates and reducing incomplete polyp resection.
Caution
Older cases did not have photo-documentation to ensure adequacy of the examination (e.g., photos of the cecum, ileocecal valve). The WEO criteria also have limitations. Some of the “new cancers” that were diagnosed >4 years after negative colonoscopy developed from missed polyps at the index exam. Also, a small portion of the CRCs that occurred within 48 months of the index exam were most likely tumors with mismatch repair mutations (i.e., Lynch Syndrome) and grew rapidly as opposed to arising from missed lesions. Notably, Kaiser-Permanente instituted universal screening of CRCs for mismatch repair mutations in 2013.
My Practice
As seen in this study, missed lesion is the most common explanation for PCCRCs diagnosed within 48 months after a clearing or negative colonoscopy. This finding highlights the need for careful inspection of the colon during withdrawal. There are several tools and techniques that I use to optimize lesion detection during withdrawal. First, it is critical to use a high-definition colonoscope with image enhancement (e.g., narrow band imaging) capabilities to help detect and evaluate subtle lesions. Second, it is important to have a mindset for detecting flat polyps since these lesions are often missed. Third, I maximize mucosal exposure by “working the folds” (i.e., deflecting the tip of the colonoscope into the inner-haustral valley and exposing the proximal sides of each haustral folds), cleaning and suctioning any stool debris, and distending the lumen adequately. Fourth, I perform 2 or 3 passes in the right colon since adenomas are often missed in this location. Lastly, when available, I often use a distal attachment device such as a clear translucent cap to help expose the proximal sides of each haustral fold and improve mucosal exposure.
In addition to missed lesions, incomplete resection of a colorectal lesion is another common explanation for PCCRCs diagnosed within 48 months after a negative colonoscopy. There are several tips and techniques that I share with my fellows to ensure complete polyp resection. First, never tackle a polyp you cannot finish during your assigned time slot. Second, consider referral of any complex polyp to a colleague or referral center that specializes in advanced tissue resection. Third, depending on the size of the lesion, aim to remove the polyp en bloc rather than in a piecemeal fashion. Fourth, embrace the cold snare over cold forceps for polyps 10 mm or less. Fifth, I recommend using snare tip soft coagulation after endoscopic mucosal resection (EMR) of large non-pedunculated polyps >20 mm in size. Sixth, carefully inspect the piecemeal EMR defect and remove any residual or visible islands using hot forceps avulsion. Lastly, I recommend close surveillance (i.e., 6 months) for all patients after a piecemeal EMR or an ESD.
For Future Research
Larger studies evaluating the root cause of PCCRC cases are needed, particularly PCCRC cases diagnosed after 4 years following a negative colonoscopy. In addition, future studies should focus on whether patient- or system-related failures (e.g., patient refusal to follow-up for a surveillance colonoscopy) are contributing to PCCRC cases. Also, future studies should also assess the frequency of mismatch repair mutations, which can produce rapidly growing CRC, among PCCRCs.
Conflict of Interest
Dr. Lee was a co-author and investigator of this study.
@douglascorley
@jeffleemd
REFERENCES
- Lin JS, Piper MA, Perdue LA, et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016;316:545.
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564-2575.
- Rutter MD, Beintaris I, Valori R, et al. World Endoscopy Organization consensus statements on post-colonoscopy and post-imaging colorectal cancer. Gastroenterology. 2018;155:909-925.
- Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370(14):1298-1306.
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362(19):1795-1803.
- Shaukat A, Rector TS, Church TR, Lederle FA, Kim AS, Rank JM, Allen JI. Longer Withdrawal Time Is Associated With a Reduced Incidence of Interval Cancer After Screening Colonoscopy. Gastroenterology 2015 Oct;149(4):952-7.
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81(1):31-53.
- Anderson R, Burr NE, Valori R. Causes of post-colonoscopy colorectal cancers based on World Endoscopy Organization system of analysis. Gastroenterology 2020;158:1287-1299.
- Beaton D, Beintaris I, Rutter MD. Utilization and reproducibility of World Endoscopy Organization post-colonoscopy colorectal cancer algorithms: retrospective analysis. Endoscopy 2022 Mar;54(3):270-277.
- Aerts R, Severi C, Van Roey G, Harlet R, T’Syen M, Claessens C, Van Gool S, Croonen C, Janssens J. A single-centre analysis of post-colonoscopy colorectal cancer. Acta Gastroenterol Belg 2021 Jul-Sep;84(3):401-405.


