Time to Simplify ADR Calculation for Colonoscopy Quality Reporting

Jeffrey Lee, MD, MPH
Research Scientist and Attending Gastroenterologist, Kaiser Permanente San Francisco Medical Center, San Francisco, CA
This summary reviews Corley DA, Jensen CD, Chubak J, et al. Evaluating different approaches for calculating adenoma detection rate: is screening colonoscopy the gold standard? Gastroenterolgy 2023;165(3):784-787.e4.
Access the article through PubMed
Correspondence to Jeffrey Lee, MD, MPH. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Do adenoma detection rates (ADRs) calculated by different indications (especially overall ADR using all colonoscopies vs screening ADR using only screening colonoscopies) have comparable associations with post-colonoscopy colorectal cancer (PCCRC)?
Design: Retrospective cohort study.
Setting: Four community-based health care systems in the United States (Kaiser Permanente Northern California, Kaiser Permanente Southern California, Kaiser Permanente Washington, and Parkland Hospital/University of Texas Southwestern).
Patients: In total, 1,046,916 patients had a negative colonoscopy (i.e., negative for colorectal cancer [CRC]) performed by 487 physicians from 2011-2019.
Exposure: The ADR of each patient’s physician based on screening, colon polyp surveillance, and diagnostic examinations (including positive fecal immunochemical tests) in the calendar year prior to the patient’s negative colonoscopy. In addition, overall ADR of each patient’s physician was based on all colonoscopy indications.
Outcome: The primary outcome was PCCRC, diagnosed at least 6 months after any negative colonoscopy (all indications).
Data Analysis: ADRs calculated as medians with interquartile ranges. Risk of PCCRC based on median ADR was calculated with Cox proportional hazards regression.
Results: The median ADRs and interquartile ranges for overall ADR was 36.3% (29.2%–44.4%); screening ADR: 29.7% (22.4%–38.1%); diagnostic ADR: 37.1% (30.6%–44.5%); and, surveillance ADR: 48.6% (38.8%–58.5%). The median overall ADR was an absolute 6.6% higher than the median screening ADR (P < .01) in a comparison of paired ADR values for each physician. ADRs across colonoscopy indications (i.e., screening, surveillance, diagnostic, and overall) were similarly inversely associated with PCCRCs (Figure 1). For patients of physicians with overall ADRs of 45% versus <25%, the hazard ratio (HR) for PCCRC risk was 0.44 (95% confidence interval [CI]: 0.35–0.55). Similarly, for patients of physicians with screening ADRs of 45% versus <25%, the HR for PCCRC risk was 0.43 (0.32–0.59). Although ADR ranges within quartiles varied by indication, comparable fourth vs first quartile associations with PCCRC risk were found across all indications (e.g., overall ADR versus screening ADR, 0.45 [0.36–0.55] versus 0.47 [0.38–0.57], respectively).
Funding: National Cancer Institute / National Institutes of Health
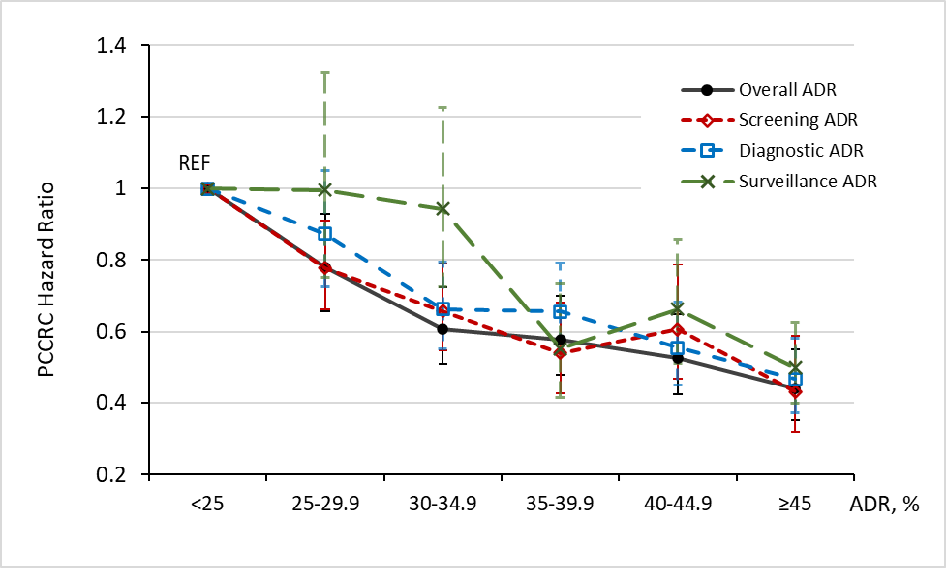 Figure 1. Associations between adenoma detection rates (ADR) quartiles and risk of post-colonoscopy colorectal cancer (PCCRC). Reprinted from Gastroenterolgy, 165(3), Corley et al. Evaluating different approaches for calculating ade-noma detection rate: is screening colonoscopy the gold standard? PP 784-787.e4. Copyright 2023 with permission from Elsevier.
Figure 1. Associations between adenoma detection rates (ADR) quartiles and risk of post-colonoscopy colorectal cancer (PCCRC). Reprinted from Gastroenterolgy, 165(3), Corley et al. Evaluating different approaches for calculating ade-noma detection rate: is screening colonoscopy the gold standard? PP 784-787.e4. Copyright 2023 with permission from Elsevier.
COMMENTARY
Why Is This Important?
The beneficial effect of colonoscopy on reducing CRC incidence and mortality is largely derived from early detection and removal of adenomas.1 Studies have shown the magnitude of this benefit varies based on the quality of the colonoscopy examination, particularly the ability to detect adenomas.2,3 Physician ADR has been widely recommended as a key colonoscopy quality metric because of its inverse association with PCCRC.4 However, this association has mainly been limited to ADR from screening colonoscopies.2,3 Although calculating ADRs from screening colonoscopies was intended to provide an “apples to apples” comparison between physicians and across practices, measuring ADR from one indication has been challenging for many health care systems and practices. Often, ascertaining colonoscopy indication may include manual chart review or utilization of natural language processing tools; this can be extremely labor intensive and subject to misclassification, especially since multiple indications (e.g., screening and rectal bleeding) may be listed for a single colonoscopy. Thus, this study fills in an important gap in colonoscopy quality measurement by testing whether ADR calculated from all colonoscopies shows similar inverse associations with PCCRC as compared with ADR calculated from only screening examinations.
Key Study Findings
Similarly, patients of physicians with screening ADRs of > 45% had a 57% reduced risk of PCCRC (HR: 0.43, 95% CI: 0.32-0.59) compared with patients of physicians with screening ADRs <25%. Nearly all endoscopists remained within the same ADR quartile regardless of whether overall or screening indication was used. This multi-center cohort study further supports the relationship between physician ADR and PCCRC, and this inverse relationship is the same regardless of whether the ADR is calculated from screening colonoscopies or from all colonoscopies. This study provides an important step to supporting a more pragmatic and less burdensome way to measure ADR for colonoscopy quality reporting.
Caution
Each institution from this study utilized different methods for capturing adenoma information and the indication for each colonoscopy.
My Practice
Over the past few years, our healthcare system has provided annual ADRs from screening colonoscopies for each gastroenterologist along with other important colonoscopy quality indicators (e.g., cecal intubation rate) to facilitate self-assessment and performance improvement. To do this, our healthcare system leveraged the electronic health record system and pathology databases to identify all colonoscopies performed by a gastroenterologist and whether an adenoma was detected. Each colonoscopy examination was then assigned an indication (e.g., screening, surveillance, or diagnostic) using a validated colonoscopy algorithm. This algorithm was designed to minimize misclassification of screening examinations by using a combination of administrative, diagnostic, and procedure codes linked with laboratory, pathology, and cancer registry data to classify colonoscopy indications. Because of the compelling findings from this study, our healthcare system has now modified its approach to calculating ADR for each gastroenterologist by using all examinations, regardless of its indication, rather than screening examinations. This has truly simplified the once time-consuming process of generating ADRs for each gastroenterologist and has minimized the concern of “indication bias.”
In addition to simplifying ADR calculation for quality improvement, there are several tips I share with my fellows and colleagues to improve adenoma detection. First, it is critical to use a high-definition colonoscope with image enhancement capabilities to help detect and evaluate subtle lesions. Second, it is important to have mindset for detecting flat polyps since these lesions are often missed. Third, I maximize mucosal exposure by “working the folds” (i.e., deflecting the tip of the colonoscope into the inner-haustral valley and exposing the proximal sides of each haustral folds), cleaning and suctioning any stool debris, and distending the lumen adequately. Fourth, I perform 2 or 3 passes in the right colon since adenomas are often missed in this location. Lastly, when available, I often use a distal attachment device such as a clear translucent cap to help expose the proximal sides of each haustral fold and improve mucosal exposure.
For Future Research
Additional studies are needed to develop thresholds or benchmarks (minimum and aspirational) for overall ADR. More studies are also needed to evaluate whether improvement in overall ADR over time for physicians is associated with reduced PCCRC risk. Based on these data, an ADR threshold of 35% may be appropriate if an overall ADR is calculated from all colonoscopies.
Conflict of Interest
Dr. Lee was a co-author and investigator of this study.
@douglascorley @jessicachubak @jeffleemd
REFERENCES
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016;315(23):2564-2575.
- Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370(14):1298-1306.
- Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362(19):1795-1803.
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81(1):31-53.
- Lee JK, Jensen CD, Lee A, et al. Development and validation of an algorithm for classifying colonoscopy indication. Gastrointest Endosc 2015;81(3):575-582.e4.

