NSAID Use and the Risk of IBD Exacerbations: Fact or Fiction?
 Philip N. Okafor, MD, MPH1
Philip N. Okafor, MD, MPH1
1Senior Associate Consultant, Department of Gastroenterology, Mayo Clinic, Jacksonville, FL
This summary reviews: Cohen-Mekelburg S, Tony V, Wallace B et al. The Association Between Nonsteroidal Anti-Inflammatory Drug Use and Inflammatory Bowel Disease Exacerbations: A True Association or Residual Bias? Am J Gastroenterol. 2022 Nov 1;117(11):1851-1857.
Correspondence to Philip N. Okafor, MD, MPH, Associate Editor. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Does the use of nonsteroidal anti-inflammatory drugs (NSAIDs) in patients with inflammatory bowel diseases (IBD) increase the risk of exacerbations?
Setting: This retrospective study was performed using data from patients with IBD in the Veterans Affairs (VA) Corporate Data Warehouse between January 1, 2004, to September 30, 2015.
Participants: An initial analysis cohort of patients with Crohn’s disease and ulcerative colitis was created using an algorithm of International Classification of Diseases, Ninth Revision (ICD-9) codes which required at least 2 clinical encounters for IBD with at least 1 being an outpatient visit.
Intervention/Exposure: NSAID exposure was the primary independent variable, and this was identified using outpatient pharmacy files based on dispense date. Aspirin and acetaminophen exposures were not included.
Outcomes: The primary outcome was IBD exacerbation defined as any outpatient IBD-related corticosteroid prescription requiring at least a 1-week supply without a non-IBD indication in the week preceding the date the corticosteroid prescription was filled. This allowed maximization of specificity in the ascertainment of an IBD flare.
Data Analysis: IBD patients with NSAID exposure were matched 1:1 to those without NSAID exposure based on preselected potential confounders including age, gender, race, Charlson comorbidity score, smoking status, IBD type, use of immunomodulator or biologic medications. The association between NSAID exposure and time to IBD flare in this matched cohort was assessed using a Cox proportional hazards model. Only the first exacerbation after NSAID use was studied. To evaluate for residual confounding, a previous event rate ratio was computed. To assess for within-person confounding, a self-controlled case series analysis was performed to estimate incidence rate ratios of IBD flares at a predetermined time range of hypothesized excess risk (6 months after NSAID exposure) compared to a pre-exposure time frame (1-year preceding NSAID exposure), but only in the cohort of patients that experienced an IBD flare after NSAID exposure.
Funding: The authors disclosed various funding sources including KL2TR002241 funding from the National Institute of Health, a Digestive Health grant from Glaxo-Wellcome Institute, and NIHP30DK050306 funding via a VA Health Services Research award.
Results: An analysis cohort of 35,031 patients was created after matching 15,705 patients with NSAID exposure to 19,326 patients without NSAID exposure. Most patients were male (93.2%) and White (88.8%). The mean follow-up was 5.9 years. Patients exposed to NSAIDs were more likely younger (57.1 vs 61.9 years, P <0.001) and female (91.4% vs 94.6%, P <0.001). Patients with IBD exposed to NSAIDs had a higher likelihood of IBD exacerbation compared to the unexposed (Hazard ratio [HR] 1.24; 95% confidence interval [CI] 1.16-1.33). However, the likelihood of an IBD exacerbation before NSAID exposure was 1.3 (95% CI 1.21-1.39) in the NSAID-exposed group vs the unexposed. As such, the computed previous event rate ratio of 0.95 (95% CI 0.89-1.01) raised the possibility of residual confounding.
The case series analyses of 3,968 patients with NSAID exposure that had at least 1 IBD flare-up showed an incidence rate ratio of 1.95 (95% CI 1.79-2.15) in the 1 year before NSAID exposure, 6.27 (95% CI 5.15-7.63) in the 0-to-2-week transition period following exposure, 1.77 (95% CI 1.37-1.43) in the 2 to 6 week post-NSAID dispense date, and 1.24 (95% CI 1.07-1.43) in the 6 weeks to 6 months post-NSAID dispense date. A sensitivity analysis using an alternative pre-exposure period of 1-month preceding NSAID dispense date was performed to assess for robustness of the initial case series analyses assumptions. A similar trend in incidence rate ratios was observed.
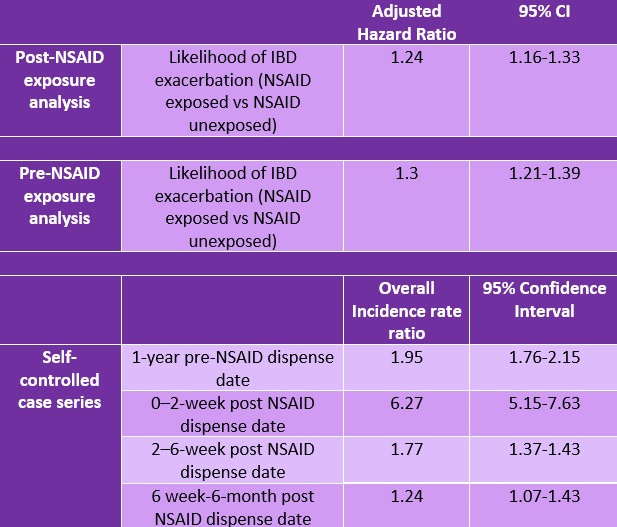
Table 1. Results of a Cox proportional hazards model on the likelihood of IBD exacerbation before and after NSAID exposure, and self-controlled case series.
CI, confidence interval; IBD, inflammatory bowel disease; NSAID, nonsteroidal anti-inflammatory drugs.
COMMENTARY
Why Is This Important?
In large studies of patients with IBD, as much as 60% report abdominal pain1, a rate that is higher than in the general population.2, 3 Abdominal pain is a hallmark presentation of IBD exacerbations, but it remains undertreated because of the concern that the use of analgesics (including NSAIDs and opiates) may exacerbate symptoms or mask a relapse.4 As such, despite the anti-inflammatory and analgesic properties of NSAIDs, many providers avoid them in patients with IBD exacerbations. Cohen-Mekelburg et al perform a retrospective multimethod analysis using VA data to investigate the association between NSAID use and IBD exacerbations. They utilize analytical methods aimed at minimizing the effects of residual confounding and reverse causality or protopathic bias in database studies (e.g., NSAIDs are prescribed for abdominal pain related to early IBD exacerbation, so it is the IBD exacerbation that causes the medicine to be prescribed, leading to an overestimate of the risk of IBD exacerbation with NSAID use in epidemiologic studies). This has been a major limitation of similar studies in the past.
Key Study Findings
While the authors show an increased risk of IBD exacerbations following NSAID exposure, they also observed that the overall likelihood of IBD exacerbation before NSAID exposure was also greater in the NSAID-exposed cohort (Table 1).
Caution
The major strengths of this study are the analytical methods used to control for reverse causality and minimize residual confounding. However, the study is limited by the difficulty generalizing the results which were obtained using VA data. Unsurprisingly, their analysis cohort was made up of 93% men, 89% White, with a mean age of 60 years. Despite this, they found that NSAID exposure was higher among younger patients and females which could suggest that different results could be seen in a younger population with more women. The authors also allude to the limited availability of details on IBD history including phenotype and duration of disease. Importantly, because NSAID can be obtained without a prescription, it can be difficult to account for their use outside the VA system which can lead to misclassification bias. The authors also did not investigate the outcome of GI bleeding which may accompany IBD exacerbations and could be impacted by NSAID use. Even with these limitations, this study provides some of the best evidence addressing a very important issue in the care of patients with IBD.
My Practice
In my practice, we tend to avoid NSAID in hospitalized patients with IBD exacerbations. This is because even though these patients may present with pain, they may also present with concurrent gastrointestinal bleeding. In addition, hospitalization affords the timely administration of IV steroids which leads to improvement in pain. The findings of this study may have a bigger impact on the outpatient management of day-to-day non-IBD pain as it does provide some reassurance for healthcare providers to prescribe NSAID for analgesia. Given these data, the risk of NSAID use in IBD patients is likely outweighed by the benefit if there is an appropriate reason for short-term use and it’s preferable to avoid opiods in these patients.
For Future Research
It is important to validate these findings in a population outside the VA, preferably one that is younger, and consists of a larger proportion of women which will allow for secondary generalizability. It would also be important to study outcomes such as gastrointestinal bleeding especially because of the increased risk of gastric ulcers in patients on concurrent NSAID and corticosteroids.
Conflict of Interest
Dr. Philip Okafor reports no potential conflicts of interest.
@ShirleyCoMekMD
@akbarwaljee
REFERENCES
- Lonnfors S, Vermeire S, Greco M, et al. IBD and health-related Quality of Life –Discovering the True Impact. J Crohns Colitis 2014;8:1281-6.
- Almario CV, Ballal ML, Chey WD, et al. Burden of Gastrointestinal Symptoms in the United States: Results of a Nationally Representative Survey of Over 71,000 Americans. Am J Gastroenterol 2018;113:1701-1710.
- Kroenke K, Price RK. Symptoms in the community. Prevalence, Classification, and Psychiatric Comorbidity. Arch Intern Med 1993;153:2474-80.
- Norton C, Czuber-Dochan W, Artom M, et al. Systematic Review: Interventions for Abdominal Pain Management in Inflammatory Bowel Disease. Aliment Pharmacol Ther 2017;46:115-125.
Download the article summary (PDF)

