In Case You Missed It
To PPI or not to PPI: Pantoprazole Prophylaxis Does Not Reduce 90-day Mortality and Clinically Significant Adverse Events in ICU Patients
Philip N. Okafor, MD, MPH1 and Alan Barkun, MD MSc2
1Senior Associate Consultant, Department of Gastroenterology, Mayo Clinic, Jacksonville, FL
2Professor of Medicine, McGill University and the McGill University Health Center, Montreal, Quebec, Canada
This summary reviews Krag M, Marker S, Perner A et al. Pantoprazole in Patients at Risk for Gastrointestinal for Gastrointestinal Bleeding in the ICU. N Engl J Med 2018; 379; 2199-2208.
Correspondence to Philip N. Okafor, MD, MPH, Associate Editor. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Does prophylactic pantoprazole reduce the risk of gastrointestinal (GI) bleeding in critically ill patients admitted to the intensive care unit (ICU)?
Setting: From January 2016 through October 2017, 33 ICUs in Denmark, Finland, the Netherlands, Norway, Switzerland, and the United Kingdom served as study sites.
Participants: Patients considered for the study were 18 years of age or older and admitted to the ICU for an acute condition with at least 1 risk factor for clinically important GI bleeding including shock, anticoagulation use, renal-replacement therapy, mechanical ventilation expected to last >24 hours, history of liver disease or ongoing coagulopathy.
Intervention/Exposure: The study was an international, multicenter, stratified, parallel-group, placebo-controlled, and blinded clinical trial. Enrolled patients were randomized to receive intravenous (IV) pantoprazole 40 mg or placebo as a single daily dose from randomization until ICU discharge or death (maximum of 90 days).
Outcomes: The primary outcome was death within 90 days of randomization. Secondary outcomes included clinically important GI bleeding (i.e. overt GI bleeding with at least 1 of the following within 24 hours of bleeding onset: spontaneous decrease in systolic blood pressure, mean arterial blood pressure, or diastolic blood pressure of 20 mmHg or more, treatment with a vasopressor or a 20% increase in vasopressor dose, decrease in hemoglobin of at least 2 g per deciliter, or transfusion of 2 or more units of packed red cells); infectious adverse events in ICU (new-onset pneumonia or Clostridioides difficile infection); serious ICU adverse reactions; acute myocardial infection; and percentage of days alive without the use of life support. Outcome data were assessed by chart review while mortality was identified using regional and national registries, or direct contact with participants or surrogates.
Data Analysis: Intention-to-treat and per protocol analyses were performed. Binary logistic regression was used to estimate the relative risk of the primary outcome adjusted for the trial site. The primary outcome in the per-protocol population was also assessed in prespecified subgroups. Dichotomous secondary outcomes were also evaluated using a binary logistic regression of the intention-to-treat population adjusted for stratification variables and predefined risk factors. Unadjusted chi-square testing for binary outcome measures was also performed. Importantly, there was no adjustment for multiple comparisons of the secondary outcomes. A 2-sided P-value of <0.05 was considered statistically significant for the primary outcome with 95% confidence intervals (CI).
Funding: Innovation Fund Denmark.
Results: During the study period, 3,298 patients were enrolled with 1,645 randomly assigned to the pantoprazole arm, while 1,653 were assigned to the placebo arm. Ninety-day vital data were obtained for 99.5% of participants. Baseline characteristics were comparable in both groups except for chronic lung disease, coagulopathy, and emergency surgery. At 90 days after randomization, no difference was seen in mortality rate, 31.1% (n=510) in the pantoprazole group vs 30.4% (n=499) in the placebo group (relative risk [RR] = 1.02; 95% CI 0.91-1.13, P=0.76). In addition, no difference was seen between both groups for the composite secondary outcome of clinically important ICU events, 21.9% (n=360) in the pantoprazole group vs 22.6% (n=372) in the placebo group (RR = 0.96, 95% CI 0.83-1.11). While fewer patients in the pantoprazole group had a clinically important GI bleed compared to the placebo group (2.5% vs 4.2%; RR = 0.58, 95% CI: 0.40-0.86), the absence of correction for multiple comparisons limited the interpretation of the relative risk. Results were similar with adjustment for baseline risk factors and in the per-protocol population. The proportions of patients in either group with the other secondary outcomes and with single components of the composite outcome were similar between groups.
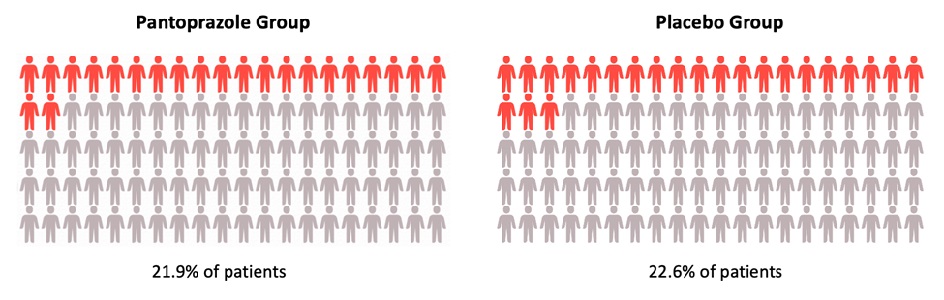
Figure 1: Death by 90 days after randomization to pantoprazole and placebo group (relative risk, 1.02; 95% confidence interval, 0.91 to 1.13).
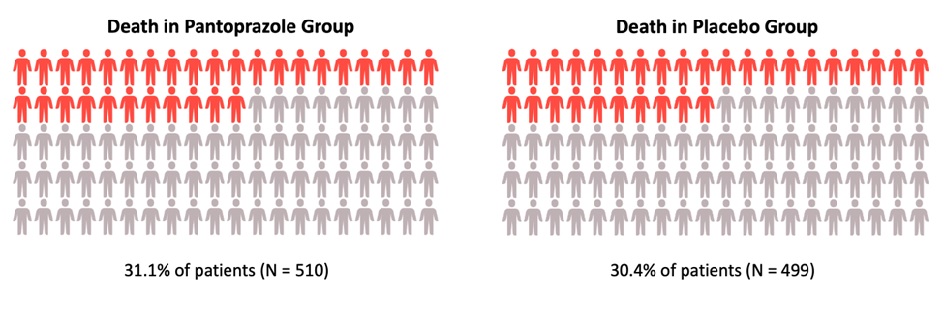
Figure 2: Occurrence of at least 1 clinically important intensive care unit event (relative risk, 0.96; 95% CI, 0.83 to 1.11).
COMMENTARY
Why Is This Important?
It is estimated that 2.5% of adults admitted to the ICU develop upper GI bleeding.1 Historically, antisecretory therapies including proton pump inhibitors (PPI) or histamine-2 receptor blockers (H2RB) have been used for stress ulcer prophylaxis.2 However, the quantity and quality of the evidence supporting stress ulcer prophylaxis is low.3 Results of the landmark Proton Pump Inhibitors vs Histamine-2 Receptor Blockers for Ulcer Prophylaxis Treatment in the Intensive Care Unit (PEPTIC) trial did not show any difference in in-hospital mortality among ICU patients receiving either PPI or H2RB, although clinically important upper GI bleeding was reported in fewer patients in the PPI group (1.3% vs 1.8%, RR = 0.73; 95% CI: 0.57-0.92).4 Other trials have also reported similar findings.3,5 Recently, PPI use has been associated with infection-related complications in the ICU,6 raising a debate about the benefits vs risks of PPI prophylaxis in the ICU. This international multicenter study by Krag et al attempts to provide more evidence on the utility of proton pump inhibitors in the ICU for the prevention of clinically significant outcomes.7
Key Study Findings
It is important to first note that the overall rate of clinically significant GI bleeding in the ICU was low in general.
Caution
While this study by Krag et al suggests that PPI prophylaxis does not impact ICU outcomes of mortality, comparable with the results of the PEPTIC trial, it is important to emphasize that the trial was not powered to detect differences in certain outcome measures, including the subgroup analyses. The GI bleeding rate of 4.2% in the placebo group was higher than the 2.5% observed in the pantoprazole group. Unfortunately, no P-value was computed because no adjustment for multiple comparisons was performed. The study design also did not mandate diagnostic endoscopy to assess the source of the bleeding.
Importantly, the authors also allude to the fact that the 5%-point difference in 90-day mortality that the study was powered for might be considered large. Finally, a sub-group analysis based on receipt of enteral nutrition which could have impacted the outcomes was also not performed.
My Practice
We have not systematically examined the patterns of PPI use for prophylaxis among our ICU patients. However, anecdotally, practice patterns vary among ICU healthcare providers with stress ulcer prophylaxis for ICU patients still being commonly prescribed. This may stem from the fact that GI bleed risk assessment is yet to be standardized,8 and as such, ICU providers would initiate prophylaxis based on their subjective risk assessment. We also routinely initiate enteral feeding via nasojejunal tubes as early as possible, which may play a role in reducing GI bleed risk in our patient population.
For Future Research
More research is needed to standardize GI bleed risk assessment among patients admitted to the ICU.8 Not only would this help define the highest risk cohorts that may indeed benefit from stress ulcer prophylaxis, but this improved risk stratification could be incorporated in future trials’ study design to make results more clinically relevant. In addition, the composite secondary outcomes used in this study (comparable to those in the PEPTIC trial) have been described as difficult to interpret and unvalidated.8 Future studies exploring the impact of PPI use on ICU outcomes should also consider composite outcomes that may be more similar in pathophysiological mechanisms.
Conflict of Interest
Drs. Okafor and Barkun reported no potential conflict of interest.
REFERENCES
- Krag M, Perner A, Wetterslev J, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med 2015;41:833-45.
- Ye ZK, Liu Y, Cui XL, et al. Critical Appraisal of the Quality of Clinical Practice Guidelines for Stress Ulcer Prophylaxis. PLoS One 2016;11:e0155020.
- Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med 2014;40:11-22.
- Australian PIft, New Zealand Intensive Care Society Clinical Trials Group AHSCCSCN, the Irish Critical Care Trials G, et al. Effect of Stress Ulcer Prophylaxis With Proton Pump Inhibitors vs Histamine-2 Receptor Blockers on In-Hospital Mortality Among ICU Patients Receiving Invasive Mechanical Ventilation: The PEPTIC Randomized Clinical Trial. JAMA 2020;323:616-626.
- Alhazzani W, Alshamsi F, Belley-Cote E, et al. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med 2018;44:1-11.
- Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology 2017;153:35-48.
- Krag M, Marker S, Perner A, et al. Pantoprazole in Patients at Risk for Gastrointestinal Bleeding in the ICU. N Engl J Med 2018;379:2199-2208.
- Barkun A, Bardou M. Proton-Pump Inhibitor Prophylaxis in the ICU – Benefits Worth the Risks? N Engl J Med 2018;379:2263-2264.


