Semaglutide Produces Mean Weight Loss of 34 Pounds over 68 Weeks in Obese and Overweight Individuals-A Huge “STEP” in Medical Management of Obesity


Sonali Paul, MD, MS 1 and Philip Schoenfeld, MD, MSED, MSc (Epi), FACG 2
1Gastroenterology Section, John R. Dingell VA Medical Center, Detroit, Michigan
2Associate Professor of Medicine, Division of Gastroenterology, Hepatology & Nutrition, Department of Medicine, The University of Chicago, Chicago, Illinois
Correspondence to Sonali Paul, MD, MS. Email: Evidence.Based.GI@gmail.com
This article reviews Wilding JPH, et al. for the Semaglutide Treatment Effect in People with Obesity (STEP) Investigators. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021; 384: 989-1002. https://www.nejm.org/doi/10.1056/NEJMoa2032183
Structured Abstract
Question: Is high-dose semaglutide (Wegovy®), a GLP-1 analogue used for Type II diabetes mellitus (DM), effective and safe for sustained weight loss when used with lifestyle interventions.
Design: Randomized, double-blind, placebo-controlled trial. Randomized in 2:1 ratio for semaglutide vs placebo.
Setting: One hundred twenty-nine sites in 16 countries in South and North America, Asia, and Europe.
Patients: There were 1961 adults with either BMI > 30 or BMI > 27 plus > 1 weight-related condition (e.g., hypertension, obstructive sleep apnea, cardiovascular disease, etc.) with approximately 75% White, 74% women, 44% Pre-diabetics and mean body weight of 230 pounds. Exclusion criteria included diabetes, history of acute pancreatitis in past 6 months, chronic pancreatitis, and previous obesity surgery.
Exposure/Intervention: Weekly subq semaglutide vs placebo injected in pre-filled pen injector plus lifestyle intervention defined as individual counseling sessions to improve adherence to reduced-calorie diet and increased physical activity. Initial semaglutide dose was 0.25mg per week and was increased every 4 weeks to reach goal dose of 2.4mg per week by week 16.
Outcome: Co-primary endpoints of mean percentage reduction in body weight and proportion of individuals with > 5% reduction in body weight from baseline at week 68.
Data Analysis: Intention-to-treat and per-protocol analysis reported.
Funding: Novo Nordisk, manufacturer of semaglutide, designed and executed the study as well as funding the study.
Results: Mean reduction in body weight was 14.9% with semaglutide plus lifestyle intervention vs 2.4% with placebo plus lifestyle intervention. At 68 weeks, mean total reduction in weight was greater with semaglutide vs placebo: 33.7 pounds vs 5.7 pounds (Figure 1). Significantly more patients in semaglutide group achieved at least 5% reduction in body weight (86.4% vs 31.5%), 10 % reduction in body weight (69.1% vs 12.0%), or 15% reduction in body weight (50.5% vs 4.9%). Nausea (44.2% vs 17.4%) and diarrhea (31.5% vs 15.9%) were more common in the semaglutide group vs placebo and discontinuation of medication due to GI side effects was also higher in semaglutide group (4.5% vs 0.8%).
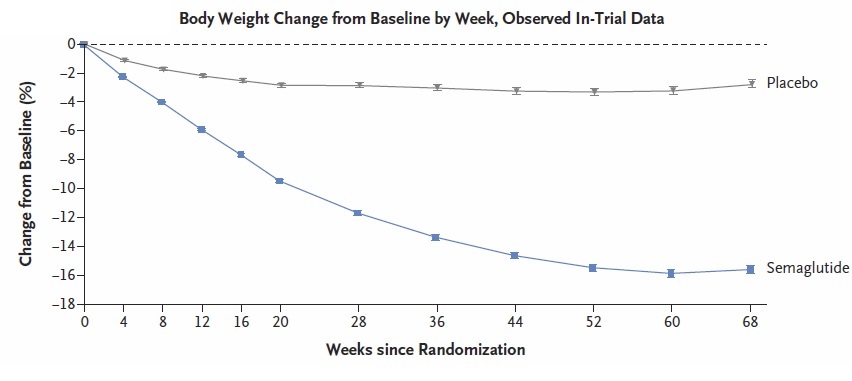
Figure 1. Body Weight Change by WeekAdapted from N Engl J Med 2021;384
Commentary Why is this important? Obesity is a global health epidemic, and the only treatment associated with long-term and sustained weight loss is bariatric surgery or endoscopic sleeve gastrectomy, which usually produce about 20% reduction in body weight.1 These data indicate that semaglutide (Wegovy®) is far superior to currently available weight loss medications.2 For example, naltrexone/buproprion (Contrave®) produces about 5% body weight reduction and phentermine/topiramate (Qysmia®) is associated with about 8% reduction. This is a true breakthrough. Although endocrinologists and other providers certified in obesity medicine are providing much of obesity management right now, gastroenterologists are frequently asked about weight loss, becoming certified in obesity medicine, and performance of bariatric endoscopy is advancing.
Key study findings: Mean reduction in body weight of almost 15%, which equates to mean loss of almost 34 pounds, was found in the semaglutide group and maintained over 68 weeks. This is far superior to weight loss observed with any other medication. Approximately 1/3 of semaglutide patients achieved 20% body weight reduction, which is similar to weight reduction with bariatric surgery or endoscopic sleeve gastrectomy.
Caution: GI adverse events, specifically nausea (44.2%) and diarrhea (31.5%), clearly occur frequently, although only about 5% of semaglutide-treated patients discontinued medication due to these GI adverse events. In our practice, patients usually develop clinically important nausea or diarrhea within the first few weeks of use and these symptoms resolve with discontinuation of medicine. Rapid weight loss is associated with developing cholelithiasis, which occurred in about 2% of semaglutide-treated patients. Although commercial insurance and Medicare Part D, frequently covers semaglutide (Wegovy®), it’s not available for obese Medicaid patients. Remember-the treatment should be combined with counseling from dietiticians on reduced calorie diets and increased physical activity. Insurance coverage for dietiticians is variable unless the patient also has diabetes.
My practice: In Dr. Paul’s hepatology practice, which includes many NASH patients, most obese and overweight patients with one additional risk factor are prescribed semaglutide. Currently, this practice has become so popular shortages are occurring at some pharmacies. We educate patients that mild nausa/diarrhea may occur as dose is escalated and that medication can be discontinued if symptoms become severe. All of our patients must also see a dietitian for counseling.
For future research: Better data across all racial/ethnic groups, in men, and in obese diabetic patients will be helpful. For gastroenterologists, more data about combination therapy with bariatric endoscopy from well-designed, prospective studies will be crucial for optimal management of obesity. With the rapidly rising prevalence of non-alcoholic steatohepatitis (NASH), semaglutide (Wegovy®) may be particularly helpful in this GI population, and a future summary will review a separate placebo-controlled, double-blind RCT that assessed efficacy of semaglutide to reduce fibrosis scores in NASH patients.
References
- Garvey WT, Mechanick H, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of paitents with obesity. Endocr Pract 2016; 22: Suppl 3: 1-203.
- Tak YJ, Lee SY. Long-term efficacy and safety of anti-obesity treatment: where do we stand? Curr Obes Rep 2021; 10: 14-30.

