In Case You Missed It
Lynch Syndrome: An Aspirin a Day Keeps Colorectal Cancer Away!

Swati G. Patel, MD, MS
Associate Professor of Medicine, University of Colorado Anschutz Medical Center; Rocky Mountain Regional Veterans Affairs Medical Center, Denver, Colorado
This summary reviews Burn J, Sheth H, Elliot F, et al for CAPP2 Investigators. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomized, placebo-controlled trial. The Lancet 2020;395:1855-63.
Access the article through PubMed
Correspondence to Swati G. Patel, MD, MS. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Does aspirin decrease the risk of colorectal cancer (CRC) in Lynch Syndrome patients?
Study Design: Double-blind, placebo controlled, randomized controlled trial (RCT).
Setting: Forty-two international centers.
Patients: Eight-hundred sixty-one individuals with Lynch Syndrome from Europe (82%), Australasia (13%), Africa (4%) and the Americas (<1%).
Intervention: Aspirin 600 mg versus placebo between 1999-2005 for 25 months.
Outcomes: The primary endpoint was time to first occurrence of CRC. CRC incidence and non-colorectal Lynch-associated cancer incidence at 10-year follow up (20-year follow up was available for participants from England, Finland, and Wales).
Data Analysis: Intention-To-Treat and Per-Protocol Analyses were performed. For time to first occurrence of CRC, life-table methods, Cox proportional hazards adjusted for age and gender, and Kaplan-Meier curves were used.
Funding: Cancer Research UK, European Union, MRC, NIHR, Bayer Pharma AG, Barbour Foundation.
Results: There was no significant difference between the placebo and intervention arm in terms of geography, genotype, or other baseline characteristics.1 Mean follow up was 10 years approximating 8,500 person-years. There was a lower proportion of patients who developed CRC in the aspirin group vs placebo group (9% vs 13%). In the intention-to-treat analysis, there was a significantly reduced hazard ratio (HR) for developing CRC in aspirin group vs placebo: HR 0.65, 95% confidence interval (CI) 0.43–0.97, P=0.035, with risk diverging starting at 5 years (Figure). Accounting for those with multiple primary CRCs, the incidence rate ratio (IRR) was 0.58, 95% CI 0.39–0.87, P=0.0085. For those who were adherent to aspirin over the study period (per-protocol analysis), the HR was 0.56, 95% CI 0.34–0.91, P=0.019, and IRR 0.50, 95% CI 0.31–0.82, P=0.0057. There was no significant difference in non-CRC Lynch associated cancers between aspirin and placebo group. There was also no significant difference in adverse event rates between the 2 groups while on treatment (cerebrovascular events: 2 out of 427 vs 3 out of 434; cardiovascular events: 1 out of 427 vs 5 out of 434; gastrointestinal bleeding: 11 out of 427 vs 9 out of 434).1
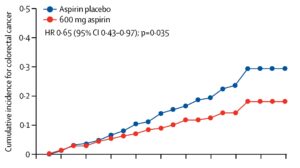
Figure 1. Cox proportional hazards (HRs and 95% CIs) comparing those on aspirin vs those on placebo and depicted by Kaplan-Meier analysis (n=861). Intention-to-treat analysis (n=427 aspirin, 434 placebo) by randomization group.
COMMENTARY
Why Is This Important?
Lynch syndrome is common, affecting every 1 out of 279 individuals,2 and is caused by a pathogenic variant in a mismatch repair gene (MLH1, MSH2, MSH6, PMS2, EPCAM). It is associated with an up to 61% lifetime risk of CRC.3 Although regular, high-quality colonoscopy and polypectomy are cornerstones for CRC prevention, post-colonoscopy CRCs occur.4 This may, in part, be due to accelerated carcinogenesis due to defective mismatch repair or due to progression via non-polypoid pathways.5 Adjuncts to colonoscopy are needed to reduce CRC risk in this common, high-risk condition. Aspirin is associated with decreased risk of CRC in the general population,6 though not routinely recommended due to overall low absolute risk in average-risk individuals, risk of short-term adverse effects, and delayed potential benefit.7 Given the high absolute CRC risk in Lynch Syndrome, aspirin use may provide adjunct benefit to this high-risk population that outweighs potential harms.
Key Study Findings
Serious adverse event rates were low in both groups, and there was no significant difference between aspirin (11 out of 427, 2.6%) and placebo (9 out of 434, 2.1%) for those who developed peptic ulcer or bleeding over the treatment period.
Caution
The study was designed in an era when high doses of aspirin (600 mg) were thought to be required for various indications (cardiovascular, etc.). Although the adverse event rates were low in the study and there was no difference between aspirin and placebo, 600 mg is a high dose and providers and patients should exercise caution and close monitoring if this high dose is used. Data from the general population suggests that lower doses may be just as protective against cancer. However, a head-to-head comparison of different aspirin dosages is not available for Lynch Syndrome patients yet. This study did not include PMS2 patients, who have a lower lifetime risk of CRC (up to 20%).3 Thus, it is unclear if they would derive the same relative benefit, and if they do, whether it is still advised to use aspirin given their absolute risk is lower than MLH1/MSH2 patients. This study kept patients on aspirin for 25 months. It is unclear what the optimal duration of therapy is.
My Practice
Based on this well-designed RCT, I discuss aspirin therapy with every Lynch Syndrome patient I see. I explain that the optimal dose is still under study. Unless there is an allergy or contraindication, I recommend adults >60 kg start 325 mg daily and monitor for any adverse effects. I also test and treat for Helicobacter pylori in all patients to minimize peptic ulcer risk. For those unable to tolerate 325 mg daily due to side effects, I encourage trial of 81 mg daily. I inform them that a dose finding study comparing 100 mg vs 300 mg vs 600 mg completed recruitment in 2019 (CAPP3). Thus, I will update them on optimal dose when those results are available. I explain that the benefit is delayed with expected benefit starting 5 years after initiating therapy. I advise patients to stay on aspirin as long as they are tolerating it but explain that this study demonstrated durable 10-year benefit after just 2 years of use. For patients with co-morbidities, on anti-thrombotics, or approaching age 60, I have an annual discussion about risks/benefits and discuss stopping it for those with limited life expectancy or where short-term risk begins to outweigh potential long-term benefit, knowing that patients will derive 10 years of protection. For my PMS2 patients, I explain that no PMS2 patients were included in this trial, thus it is unclear if there is similar benefit, especially in context of their overall lower absolute risk of CRC.
For Future Research
We will likely have 5-year outcome data from the dose finding CAPP3 study in the coming 1-2 years, which will guide what the best dose of aspirin is for our Lynch Syndrome patients. Other areas of research include understanding the mechanism of the long-term protection aspirin appears to confer. Current hypotheses include salicylate mediated modulation of the microbiome, pro-apoptotic properties of salicylate on aberrant progenitor cells or effect of salicylate on upregulation of immune surveillance.
Conflict of Interest
Dr. Patel reports no conflicts of interest.
@CaPP3
J. Burn
@GabrielaMoslein
G. Moeslein
@Adductor
T. Seppala
REFERENCES
- Burn J, Bishop DT, Mecklin JP, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med 2008;359:2567-78.
- Win AK, Jenkins MA, Dowty JG, et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol Biomarkers Prev 2017;26:404-412.
- National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Colorectal. Version 2.2023. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf Published October 30, 2023. Accessed December 7, 2023.
- Seppala T, Pylvanainen K, Evans DG, et al. Colorectal cancer incidence in path_MLH1 carriers subjected to different follow-up protocols: a Prospective Lynch Syndrome Database report. Hered Cancer Clin Pract 2017;15:18.
- Cerretelli G, Ager A, Arends MJ, et al. Molecular pathology of Lynch syndrome. J Pathol 2020;250:518-531.
- Liang PS, Shaukat A, Crockett SD. AGA Clinical Practice Update on Chemoprevention for Colorectal Neoplasia: Expert Review. Clin Gastroenterol Hepatol 2021; 19:1327-36.
- Nudy M, Cooper JL. USPSTF recommends aspirin to prevent CVD in adults 40 to 59 y as an individual decision; not recommended for adults >60 y. Ann Intern Med 2022; 175:JC98.

