Cooking Up Something New With a One-Food Elimination Diet: A Simpler Approach to Dietary Therapy for Eosinophilic Esophagitis

Jennifer M. Kolb MD, MS1 and Devin B. Patel, MD2
1Assistant Professor of Medicine
2Gastroenterology Fellow
Division of Gastroenterology, Hepatology, and Parenteral Nutrition, VA Greater Los Angeles Healthcare System, David Geffen School of Medicine at UCLA, Los Angeles, CA.
This summary reviews Kliewer K, Gonsalves N, Dellon E, et al. One-food versus six-food elimination diet therapy for the treatment of eosinophilic esophagitis: a multicentre, randomized, open-label trial. Lancet Gastro Hepatol 2023; 8: 408-21
Access the article through PubMed
Correspondence to Jennifer M. Kolb, MD, MS. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: In adults with active, symptomatic eosinophilic esophagitis (EoE), is a 1-food elimination diet (1FED) of animal milk similar to a 6-food elimination diet (6FED) of animal milk, egg, wheat, soy, nuts, and seafood, for histological remission and clinical symptoms?
Design: Multicenter, open-label, randomized controlled trial (RCT).
Settings: Ten tertiary care sites of the Consortium of Eosinophilic Gastrointestinal Disease Researchers in the United States.
Patients: Adult patients aged 18-60 years with diagnosis of EoE who were non-responders to a trial of proton pump inhibitors (PPI) were screened. Patients with active EoE symptoms and histologically active disease (defined as >15 eosinophils per high-power field (eos/hpf) in at least 1 segment among the distal, mid, and proximal regions of esophagus) during the 12-week screening period were randomized. Medications, including PPIs, were required to be maintained at same dose.
Exclusion criteria included: 1) use of topical swallowed corticosteroids within 2 months of enrollment or systemic corticosteroids within 3 months; 2) eosinophilic gastrointestinal disease beyond the esophagus; 3) gastrointestinal malabsorption disorders; 3) mild avoidance due to allergy; 4) already on dietary therapy; and, 5) previous non-response to topical corticosteroids.
Interventions/Exposure: Patients were randomly assigned in a 1:1 ratio to either 1FED or 6FED. Randomization was stratified into blocks of 4 by age (<30 years or >30), sex, and study site. This study followed an open-label design where site investigators, staff, and participants were aware of treatment allocation, however, pathologists who were assessing biopsies were blinded.
Phase 1: Participants followed the 1FED (animal milk elimination) or 6FED (animal milk, egg, wheat, soy, fish and shellfish, and peanut and tree nut elimination) for 6 weeks followed by EGD with biopsy. Individuals with treatment response (histological remission; peak eosinophil count < 15 eos/hpf) completed the study at phase 1.
Phase 2: Individuals without histological response had the option to continue into either 6FED (if failed 1FED) or topical swallowed corticosteroids (fluticasone propionate) if failed 6FED. Repeat EGD was done after 6 weeks.
Outcomes: The primary outcome was histological remission at 6 weeks, defined as peak eosinophil count <15 eos/hpf. Secondary outcomes included proportion of participants with complete remission (peak eosinophil count < 1 eos/hpf), partial remission (peak eosinophil count < 10 eos/hpf and > 6 eos/hpf) and change from baseline in peak eosinophil count. Additional secondary outcomes were histologic remission for those who completed phase 2 of the study. Additional outcomes were change in symptoms with the Eosinophilic Esophagitis Activity Index (EEsAI) and endoscopic and histologic outcomes using two validated instruments: Eosinophilic Esophagitis Endoscopic Reference Score (EREFS) and Eosinophilic Esophagitis Histology Scoring System (EoEHSS).
Data Analysis: Intention-to-treat (ITT) analysis was used to calculate the primary and key secondary endpoints. The sample size of 120 patients was calculated assuming 45% remission rates in the 1FED vs 70% remission in the 6FED. Subjects who withdrew from the study were considered non-responders and missing data was imputed with the last observation carried forward.
Funding: National Institute of Health.
Results: Between May 23, 2016, and March 6, 2019, 129 patients (mean age 37 years, 54% male) were randomly assigned to 1FED (n = 67) or 6FED (n = 62). Peak eosinophil count at baseline was higher in the 1FED vs 6FED: 50.3 vs 38.4. At 6 weeks, histologic remission was similar in the 6FED and 1FED groups (40% vs 34%, P = 0.58) (Figure 1). Similarly, there was no significant difference between the groups at stricter thresholds for histologic remission of < 6 eos/hpf (32% vs 18%, P = 0.07), although rates of complete histologic remission of < 1 eos/hpf were higher in the 6FED vs 1FED (19% vs 6%, P = 0.03). Self-reported adherence to dietary therapy was high (1FED 98%, 6FED 97%). Both groups showed improvement in endoscopic fibrostenotic measures with EREFS scores (6FED mean change –1.0 vs 1FED mean change –0.6, P = 0.28) and clinical symptoms with EEsAI (6FED mean change –8.2 vs 1FED mean change –3.0, P = 0.09). There were 21 patients who did not respond to 1FED and switched to 6FED in phase 2, of whom 43% achieved histologic remission at 6 weeks. There were 11 patients who moved on to swallowed fluticasone propionate after non-response to 6FED, of whom 82% achieved histologic remission.
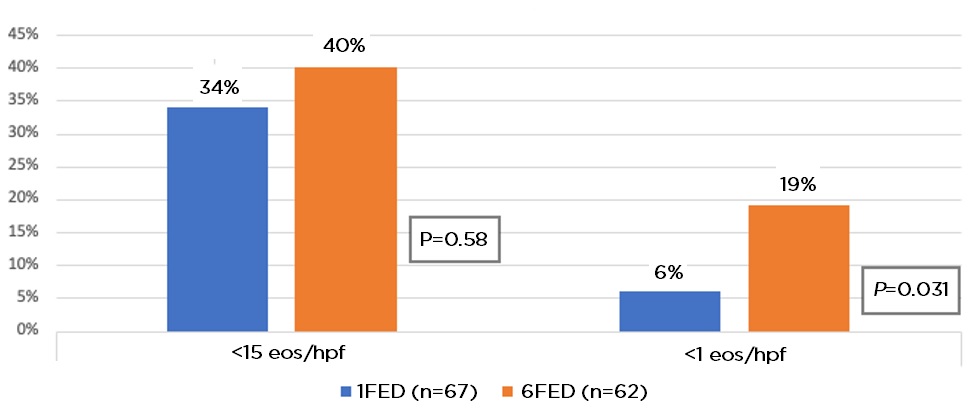 Figure 1: Primary and secondary endpoint outcomes. Proportion of patients in histological remission (<15 eos/hpf) and complete remission (<1 eos/hpf) at week 6.
Figure 1: Primary and secondary endpoint outcomes. Proportion of patients in histological remission (<15 eos/hpf) and complete remission (<1 eos/hpf) at week 6.
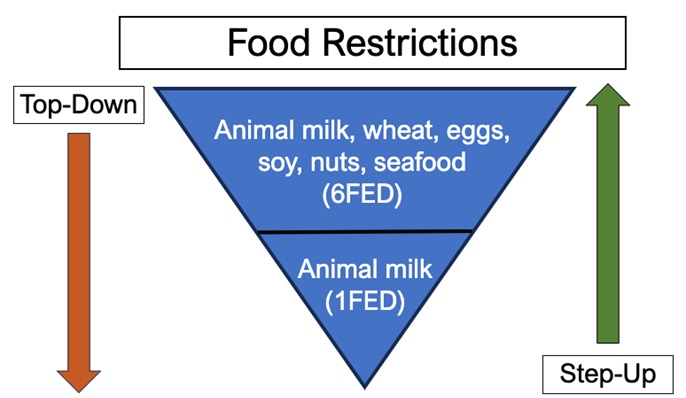
Figure 2: Initial approaches to empiric elimination diets.
COMMENTARY
Why Is This Important?
EoE is a chronic inflammatory and fibrostenotic condition driven by a food-antigen-triggered T-helper type 2 allergic immune response. Treatments include PPI, topical swallowed corticosteroids using oral inhalers approved for asthma, and more recently targeted biologic therapy with dupilumab, an interleukin-4 receptor alpha antagonist.
An alternative approach is dietary therapy, which focuses on elimination of specific food exposures thereby preventing the initiation of the inflammatory cascade. Traditionally, empiric food elimination therapy takes a top-down approach by starting with restriction of multiple food groups followed by a gradual reintroduction. There is growing interest in a step-up approach that is less restrictive and instead starts with the most allergenic food groups (animal milk) given that most patients with EoE tend to have just one or two trigger foods.3 (Figure 2).
Previous non-randomized studies have demonstrated that almost 70% of pediatric and adult patients can achieve histologic remission with an empiric 6FED1. However, 6FED poses several practical concerns that limit its utilization for treatment of EoE, including the need for frequent repeated endoscopies after a burdensome re-introduction process as well as poor patient acceptance of these long-term restrictive diets that require avoidance of commonly consumed foods.2 There have been several single-arm studies evaluating the impact of different levels of restriction with dietary elimination therapy, however comparative data is limited. This is the first randomized trial to compare 1FED to 6FED in adults with EoE.
Key Study Findings
Improvements in both histologic and endoscopic features using validated scoring systems were similar between both groups. For 1FED non-responders, 6FED was effective in 43%. In 6FED non-responders, swallowed topical steroids was effective in 82%. These findings suggest that elimination of animal milk alone is an acceptable initial dietary therapy for EoE.
Caution
Exclusion of patients who responded to PPI therapy limits generalizability of findings to a subset of the EoE population. The sample size may have been too small to identify differences in clinical symptoms and improvement in endoscopic findings with 1FED vs 6FED. Additionally, the peak eosinophil count at baseline was higher in the 1FED group (50.3) vs the 6FED group (38.4), which could have made it more difficult to achieve histologic remission in the 1FED group.
My Practice
Generally, I try to follow guidelines for management of EoE, including obtaining 6 biopsies from 2 different levels of the esophagus when screening for EoE, obtaining biopsies to check for EoE if I’m performing an EGD to manage a food impaction, and performing repeat EGD about 8-12 weeks after changing EoE treatments since improvement in dysphagia symptoms don’t always correlate with histologic remission.1-2,5
Generally, PPIs taken twice a day are my initial therapy, although I focus on shared decision making with patients and emphasize that EoE treatment is long-term and should be maintained even after dysphagia symptoms improve. For some patients, an elimination diet may be a preferred first line therapy, but strict adherence to 6FED with gradual reintroduction of potential trigger foods followed by frequent repeat EGD can be onerous for the patient. For this reason, I always explain to patients what the entire process will look like and encourage them to consider whether they will be able to follow all the recommendations. The study findings provide reassurance about starting with a 1FED, which is preferable for patients. If patients don’t achieve remission with PPIs/food elimination diets or can’t be adherent with food elimination, the decision about whether to proceed with swallowed corticosteroids or dupilumab5 should reflect the patient’s values and wishes through shared decision making.
For Future Research
More data are needed to inform the optimal duration of diet elimination therapy given the uncertainty of long-term nutritional and psychological effects. Future studies should evaluate if the current findings are relevant beyond the US since food triggers may vary geographically.
Conflict of Interest
Dr. Kolb and Dr. Patel report no potential conflicts of interest.
Note: The authors of the article published in the Lancet Gastro Hepatol are active on social media. Tag the to discuss their work and this EBGI summary:
@EvanDellon
@IkuoHirano
@ngonsalvesMD
REFERENCES
- Rank MA, Sharaf RN, Furuta GT, et al. Technical review on the management of eosinophilic esophagitis: a report from the AGA institute and the joint task force on allergy-immunology practice parameters. Ann Allergy Asthma Immunol 2020;124(5):424-440.e17.
- Hirano I, Chan ES, Rank MA, et al. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Ann Allergy Asthma Immunol 2020;124(5):416-423.
- Molina-Infante J, Arias Á, Alcedo J, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2-4-6 study. J Allergy Clin Immunol 2018;141(4):1365-1372.
- Leiman DA, Kamal AN, Otaki F, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol 2023;118: 1091-95.
- Kamal A, Schoenfeld P. Dupilumab, an anti-interleukin-4 monoclonal antibody, for Eosinophilic Esophagitis: Revising the Treatment Paradigm. Evidence-Based GI Feb 2023. https://gi.org/journals-publications/ebgi/kamal_february2023/. Accessed August 8, 2023.

