Does Choice of Anticoagulant Influence Risk of Gastrointestinal Bleeding?
 Ravy K. Vajravelu, MD, MSCE
Ravy K. Vajravelu, MD, MSCE
Assistant Professor of Medicine, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh School of Medicine; Staff Gastroenterologist, VA Pittsburgh Healthcare System, Pittsburgh, Pennsylvania
This summary reviews Ingason AB, Hreinsson JP, Agustsson AS, et al. Rivaroxaban is Associated with Higher Rates of Gastrointestinal Bleeding than Other Direct Oral Anticoagulants. Ann Intern Med 2021; 174:1493-1502. https://doi.org/10.7326/M21-1474
Correspondence to Ravy K. Vajravelu, MD, MSCE. Associate Editor. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Is rivaroxaban (Xarelto), a direct-acting oral anticoagulant (DOAC), associated with higher rates of gastrointestinal (GI) bleeding compared to other frequently used DOACs, apixaban (Eliquis) and dabigatran (Pradaxa)?
Design: Retrospective cohort study, 2014-2019.
Setting: Icelandic national healthcare system.
Patients: New DOAC users.
Interventions/Exposure: Treatment with apixaban, dabigatran, or rivaroxaban at anticoagulation-level doses.
Outcome: Clinically relevant GI bleeding. This was defined as a GI bleed that led to a medical intervention, unscheduled physician visit, or temporary cessation of anticoagulation. These were identified by International Classification of Diseases, Tenth Revision codes and manual verification through chart review.
Data Analysis: The main results of interest were the rates of GI bleeding associated with each anticoagulant. To adjust for factors that could confound the rates, the investigators used inverse probability of treatment weighting (IPTW). IPTW is a form of propensity score adjustment that estimates how likely an individual is to receive an exposure of interest (a given DOAC in this study). The likelihood of receiving the exposure is then used to weight how much each individual contributes to the study results. Individuals who are very likely to receive or not receive the exposure contribute less to the results. Because of this adjustment, the results should approximate the effect of the exposure of interest in the general population.
Funding: Icelandic Centre for Research and the Landspítali University Hospital Research Fund.
Results: Overall, 5,868 individuals met inclusion criteria. Two thousand one hundred fifty-seven (37%) were treated with apixaban, 494 (8%) with dabigatran, and 3,217 (55%) with rivaroxaban. Atrial fibrillation was the indication for anticoagulation in 80% of individuals. Investigators identified 241 GI bleeding events among the cohort. Seventy-two (30%) were upper GI bleeding, 135 (56%) were lower GI bleeding, and 34 (14%) could not be classified. One hundred forty-six (61%) of the events were classified as major GI bleeding.
Rivaroxaban was associated with the highest rate of GI bleeding at 3.2 events per 100 person-years, compared to apixaban and dabigatran at 2.5 and 1.9 events per person-year, respectively. The hazard ratio (HR) of GI bleeding for rivaroxaban and dabigatran relative to apixaban were 1.42 (95% CI 1.04-1.93) and 0.87 (0.46-1.65), respectively. In subgroup analyses, rivaroxaban was associated with higher rates of major GI bleeding and lower GI bleeding, but these were not statistically significant relative to apixaban (Table 1).
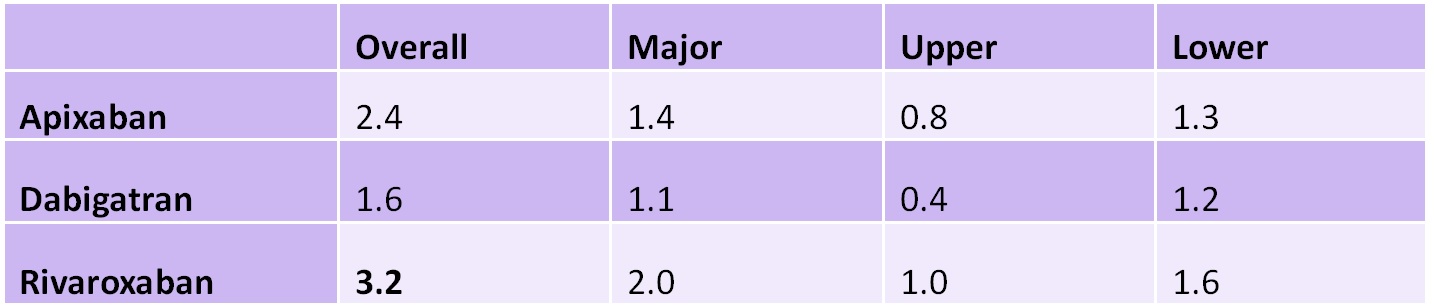
Table 1: Rates of GI bleeding per 100 person-years by DOAC
Note: Bold denotes statistically significant 95% confidence interval relative to apixaban.
COMMENTARY
Why Is This Important?
This study1 uses real-world evidence to support prior observational studies that demonstrated that rivaroxaban (Xarelto) is associated with higher GI bleeding risk compared to other DOACs.2,3 In particular, the strengths of this study include a high-quality database with very little missing data or loss to follow-up due to the nationalized healthcare system in Iceland. Furthermore, the investigators manually confirmed all cases of GI bleeding, giving confidence to the study conclusions. The findings are potentially consistent with the hypothesis that rivaroxaban has higher GI bleeding risk due to its pharmacokinetics. Rivaroxaban is dosed daily instead of twice daily like apixaban (Eliquis) and dabigatran (Pradaxa). As such, rivaroxaban achieves higher levels of factor Xa inhibition.4
Key Study Findings
Rivaroxaban was associated with slightly higher rates of GI bleeding compared to apixaban (3.2 vs 2.5 per 100 person-years, respectively). One hundred forty-two individuals would need to be treated for 1 year with apixaban instead of rivaroxaban to prevent 1 case of GI bleeding (number needed to harm). Whether this benefit is sufficient to justify potential decreased adherence from twice daily dosing should be discussed between patients and their prescribers.
Caution
Relative to prior observational studies of DOAC-associated GI bleeding, the number of individuals included in the study was relatively small. This reduces the power and precision of the analyses. Furthermore, because of the relative recency of DOAC availability, the mean follow-up time was only about 1.5 years. The outcomes described in this study should be considered short term and not necessarily representative of risk for long-term users.
My Practice
In my luminal gastroenterology practice, I most commonly encounter DOACs in preparation for elective gastrointestinal endoscopy. I recommend anticoagulation holds consistent with recent joint clinical practice guidelines from the American College of Gastroenterology and Canadian Association of Gastroenterology.5 That is, I do not recommend holding anticoagulation for low-risk procedures such as diagnostic endoscopy with mucosal biopsies. However, if the procedure is a screening colonoscopy, I usually recommend an anticoagulation hold because of the possibility of endoscopic mucosal resection of large polyps. I practice in an integrated care delivery network, so I am privileged to work with the prescribers of the anticoagulant and anticoagulation pharmacists in determining the duration of pre-procedure anticoagulation holds. In general, our recommendations align with clinical practice guidance based on renal function from the American Society for Gastrointestinal Endoscopy (Table 2).6 In the few cases where patients arrive for their endoscopy without holding anticoagulation, I discuss the risks and benefits of performing the procedure versus rescheduling. In particular, for colonoscopies performed on anticoagulation, this includes the possibility of need for a second colonoscopy to remove large polyps by endoscopic mucosal resection after the anticoagulant has been held.
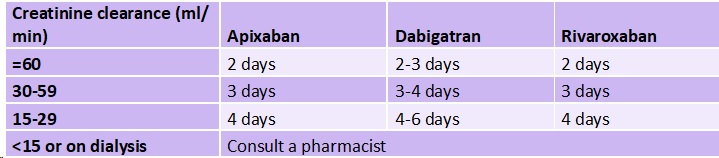
Table 2: Number of days to hold DOAC prior to endoscopy for procedures with high risk of GI bleeding
Note: Adapted from (6)
________________________
Another scenario where I encounter DOACs is during inpatient consultation for patients who require anticoagulation but have high risk of GI bleeding. Based on the results of this study and other studies demonstrating higher GI bleeding rates for rivaroxaban, I recommend apixaban over rivaroxaban for new anticoagulation starts. However, because the number needed to harm for rivaroxaban versus apixaban is relatively high at 142, I do not recommend prophylactic DOAC changes from rivaroxaban to prevent GI bleeding.
For Future Research
Resumption of anticoagulants post endoscopy is a corollary to the topic of this study. Notably, the authors of the 2022 ACG-CAG Clinical Practice Guideline on anticoagulation management did not make a recommendation on this topic based on lack of relevant high-quality evidence. Future studies that determine rates of post-endoscopy bleeding by timing of DOAC resumption may help standardize clinical practice.
Disclosures
Dr. Vajravelu has no disclosures to report. This commentary does not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
REFERENCES
- Ingason AB, Hreinsson JP, Agustsson AS, et al. Rivaroxaban Is Associated With Higher Rates of Gastrointestinal Bleeding Than Other Direct Oral Anticoagulants: A Nationwide Propensity Score-Weighted Study. Ann Intern Med 2021;174:1493-1502.
- Hernandez I, Zhang Y, Saba S. Comparison of the Effectiveness and Safety of Apixaban, Dabigatran, Rivaroxaban, and Warfarin in Newly Diagnosed Atrial Fibrillation. Am J Cardiol 2017;120:1813-1819.
- Abraham NS, Noseworthy PA, Yao X, et al. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology 2017; 152:1014-1022 e1.
- Frost C, Song Y, Barrett YC, et al. A Randomized Direct Comparison of the Pharmacokinetics and Pharmacodynamics of Apixaban and Rivaroxaban. Clin Pharmacol 2014;6:179-87.
- Abraham NS, Barkun AN, Sauer BG, et al. American College of Gastroenterology-Canadian Association of Gastroenterology Clinical Practice Guideline: Management of Anticoagulants and Antiplatelets During Acute Gastrointestinal Bleeding and the Periendoscopic Period. Am J Gastroenterol 2022;117:542-558.
- Acosta RD, Abraham NS, Chandrasekhara V, et al. The Management of Antithrombotic Agents for Patients Undergoing GI Endoscopy. Gastrointest Endosc 2016;83:3-16.

