Reinvestigating the Lack of Association Between Proton Pump Inhibitor Use and Mortality by Accounting for Reverse Causation

Ravy K. Vajravelu, MD, MSCE
Assistant Professor of Medicine, Division of Gastroenterology, Hepatology and Nutrition, University of Pittsburgh School of Medicine; Staff Gastroenterologist, VA Pittsburgh Medical Center Healthcare System, Pittsburgh, PA
This summary reviews Lo CH, Ni P, Yan Y, et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology 2022; 163:852-61.
Correspondence to Ravy K. Vajravelu, MD, MSCE. Associate Editor. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Are proton pump inhibitors (PPIs) associated with increased mortality?
Design: Prospective cohort study.
Setting: Combined data from the Nurses’ Health Study and the Health Professionals Follow-up Study.
Patients: The Nurses’ Health Study is an ongoing prospective cohort study in the United States that recruited female nurses who were ages 30–55 in 1976. The Health Professionals Follow-up Study recruited male health professionals who were ages 40–75 in 1986. For both studies, investigators obtained information from medical records and questionnaires every 2 years. The sub-cohort selected for this study was new users of PPIs.
Exposure: Self-reported PPI use.
Outcome: The primary outcome was death from any cause. Secondary outcomes were death from specific causes such as cancer, cardiovascular diseases, respiratory diseases, digestive diseases, renal diseases, neurologic diseases, and infectious diseases.
Data analysis: The association of PPI use and death was estimated using Cox proportional hazards regression to calculate a hazard ratio (HR). To reduce the possibility of protopathic bias (also called reverse causation), the investigators conducted several secondary analyses that incorporated PPI-use lag windows (see Why Is This Important section below).
Two-year, 4-year, and 6-year lag times were assessed. For example, in a 4-year lag-time analysis, this means exposure to PPI had to be self-reported in the biennial questionnaires at least 4 years before death occurred.
Funding: National Institutes of Health and the Crohn’s and Colitis Foundation.
Results: Out of 71,887 study participants, 22,125 died during follow-up, of which 2033 (10.1%) were PPI users at the time of death. In the analysis that did not account for lag time, PPI use was associated with mortality from all-causes, cancer, cardiovascular diseases, respiratory diseases, digestive diseases, and renal diseases. There was no association with neurologic or infectious diseases. By incorporating progressively longer lag-times, the investigators demonstrated that the association of PPI use with each cause of death was nullified, except for the association of PPI use and death from renal causes (HR 2.45, 95% confidence inter-val (CI) 1.59 – 3.78 in the 6-year lag analysis) (Figure 1).
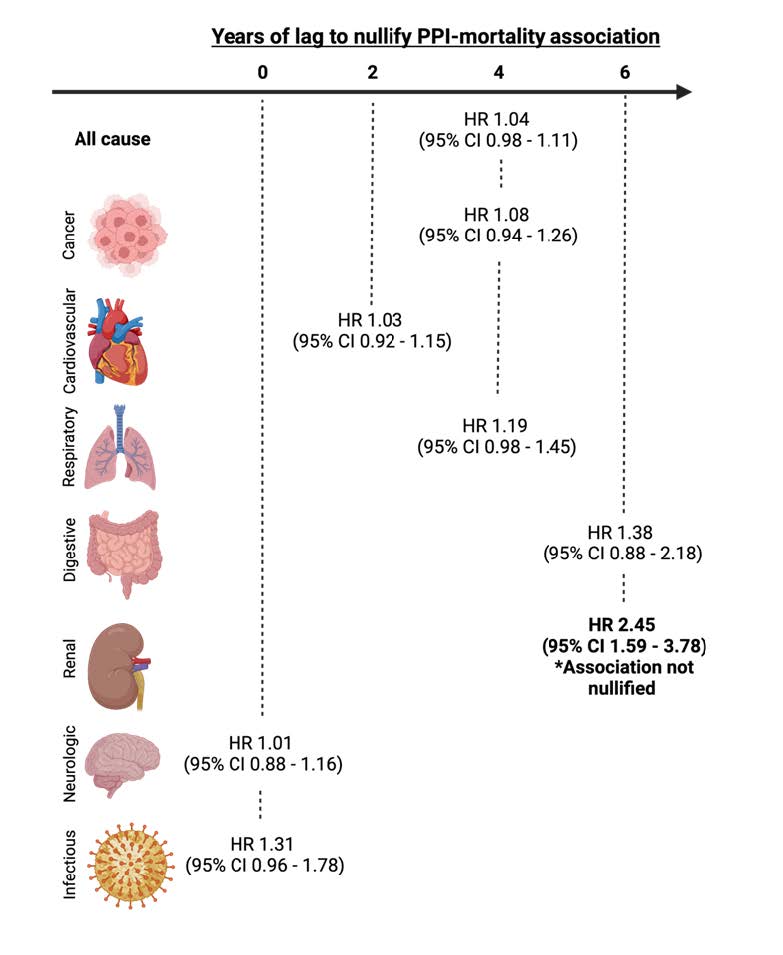
Figure 1: Years of lag time to nullify statistically significant association between proton pump inhibitor (PPI) use and mortality. Nullification of association implies that protopathic bias con-tributes to spurious associations. Image created with BioRender.com. CI, confidence interval; HR, hazard ratio.
COMMENTARY
Why Is This Important?
PPIs are one of the most frequently used medications in the United States.2,3 Recently, several retrospective studies have linked PPIs to adverse effects, such as chronic kidney disease, dementia, and death.4-6 However, there are concerns about the validity of these conclusions due to methodologic limitations of the studies7-9, including inadequate adjustment for protopathic bias, which occurs when patients receive an exposure of interest to treat prodromal symptoms of an impending outcome.
A common example helps explain protopathic bias, which is also called reverse causation. Imagine a patient who presents to their primary care physician for assessment of atypical chest pain. The patient receives a PPI for presumptive treatment of gastroesophageal reflux disorder (GERD), but the patient later develops a fatal myocardial infarction because the chest pain was truly angina secondary to coronary artery disease. If a retrospective study including this patient does not account for protopathic bias, the PPI would be associated with the death, which was actually caused by coronary artery disease. To reduce the influence of protopathic bias in the current study results, the investigators incorporated lag windows so that PPI use was only considered after sufficient time had passed from initiation.
Randomized controlled trials (RCTs) are the standard for demonstrating causation, and COMPASS, a recent double-blind, placebo-controlled RCT of over 17,000 individuals with chronic cardiovascular disease followed for a mean duration of 3 years, only found an in-creased risk of enteric infections with PPI use. There are some remaining details to be investigated based on the limitations of the study10, including the relatively short length of follow-up and limited generalizability of the study population. Reassurance about the safety of PPIs also comes from two recent retrospective cohort studies corroborated the findings of COMPASS by demonstrating no association between PPI use and mortality using data from the U.S. Medicare system and the UK Biobank.7,11 This study from Lo et al adds to these reassuring results by incorporating lag times of 2, 4, and 6-years of PPI use to adjust for protopathic bias.
After accounting for protopathic bias, PPI use was not associated with death from all-causes, cancer, cardiovascular disease, respiratory disease, digestive disease, neurologic disease, or infectious diseases. There was an association between PPI use and death from renal disease.
Caution
Because PPI use was assessed only every 2-years, the lag-time windows are long. Excluding events in 2-year increments reduces the statistical power of the analysis to identify conditions that confer small increased risk in mortality. Additionally, the authors did not implement competing-risks approaches for the analysis of the secondary mortality outcomes. This could bias the conclusions of the study. Finally, this study only investigated mortality. The lack of association between cause-specific mortality and PPI use does not necessarily mean that the PPI use is not associated with the condition itself—especially if the condition does not usually lead to death.
My Practice
I prescribe PPIs frequently in my luminal gastroenterology practice at a Veterans Affairs Health System. The most common indications are chronic GERD, chemoprevention of Barrett’s esophagus progression, and eosinophilic esophagitis. For patients who will use PPIs longer than eight weeks, I counsel about the state of the PPI adverse effects literature. In particular, I summarize the concerns raised by early retrospective cohort studies and mention that there were methodologic issues with many of them. I then summarize the results of the COMPASS randomized control trial, which did not find any association between PPI and fractures, diabetes, COPD, dementia, cancer, chronic kidney disease, etc., and relay that more recent, high-quality retrospective cohort studies have corroborated it. Finally, I acknowledge that there still may be risks to long-term PPI use, in particular risk of enteric infections as identified by COMPASS and risk of renal disease as demonstrated in this study. As such, I assure patients that we will periodically reassess their need for chronic PPIs and maintain them on the lowest effective dose.
For Future Research
The mechanism of action for the association between PPI use and renal disease is often hypothesized to be secondary to acute interstitial nephritis. Further characterization of this relationship and whether it mediates the association between PPI use and renal mortality is warranted.
Conflicts of Interest
Dr. Vajravelu reports no conflicts of interest. He is an employee of the Department of Veterans Affairs. This commentary does not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
@AndyChanMD
@ChunHanLoMDMPH
REFERENCES
- Lo C-H, Ni P, Yan Y, et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology 2022;163:852-861.e2.
- Mishuk AU, Chen L, Gaillard P, et al. National trends in prescription proton pump inhibitor use and expenditure in the United States in 2002-2017. J Am Pharm Assoc (2003) 2020.
- Number of people with purchase by prescribed drug, United States, 1996 to 2019. Medical Expenditure Panel Survey: Agency for Healthcare Research and Quality.
- Xie Y, Bowe B, Yan Y, et al. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ 2019;365:l1580.
- Vilcu AM, Sabatte L, Blanchon T, et al. Association Between Acute Gastroenteritis and Continuous Use of Proton Pump Inhibitors During Winter Periods of Highest Circulation of Enteric Viruses. JAMA Netw Open 2019;2:e1916205.
- Gomm W, von Holt K, Thome F, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol 2016;73:410-6.
- Baik SH, Fung KW, McDonald CJ. The Mortality Risk of Proton Pump Inhibitors in 1.9 Million US Seniors: An Extended Cox Survival Analysis. Clin Gastroenterol Hepatol 2022;20:e671-e681.
- Hayes KN, Nakhla NR, Tadrous M.
Further Evidence to Monitor Long-term Proton Pump Inhibitor Use. JAMA Netw Open 2019;2:e1916184. - Corley DA. Safety and Complications of Long-Term Proton Pump Inhibitor Therapy: Getting Closer to the Truth. Gastroenterology 2019;157:604-607.
- Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology 2019; 157:682-691.
- He Q, Xia B, Meng W, et al. No Associations Between Regular Use of Proton Pump Inhibitors and Risk of All-Cause and Cause-Specific Mortality: A Population-Based Cohort of 0.44 Million Participants. Am J Gastroenterol 2021;116:2286-2291.
Download the Article Summary (PDF)

