Prophylactic Rifaximin Decreases Post-TIPS Hepatic Encephalopathy

Philip Schoenfeld, MD, MSEd, MScEpi, FACG
Chief Emeritus-Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, Michigan
This summary reviews: Bureau C, Thabut D, Jezequel C, et al. The Use of Rifaximin in the Prevention of Overt Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt. Ann Intern Med 2021; 174: 633-40.
Correspondence to Dr. Philip Schoenfeld, Editor-in-Chief. Email: EBGI@gi.org
Read the article through PubMed
STRUCTURED ABSTRACT
Question: Does rifaximin 600mg bid prevent overt hepatic encephalopathy (HE) after transjugular intrahepatic portosystemic shunt (TIPS) compared to placebo?
Design: Multi-center, double-blind, placebo-controlled randomized controlled trial. Randomization stratified based on presence or absence of prior episode of overt HE and to Child-Pugh class (A + B or C).
Setting: Twelve tertiary care centers in France. Patients were recruited by expert hepatologists at each site.
Patients: Included patients were: (a) > 18 years old; and, (b) planning to have elective TIPS for intractable ascites or to prevent variceal rebleeding due to cirrhosis. Exclusion criteria included recurrent or persistent overt HE, hepatocellular carcinoma beyond Milan criteria, or Child-Pugh score > 12.
Interventions/Exposure: Rifaximin 600 mg twice a day vs identical placebo tablets, starting 2 weeks prior to scheduled TIPS and continued for 168 days post-TIPS. All patients were treated with 10 mm covered stents. Prophylaxis for overt HE with lactulose was not allowed, but could be used for episodes of overt HE.
Outcome: The primary endpoint was cumulative incidence of overt HE, defined by West Haven modified criteria, which also defines isolated asterixis as Grade 2 overt HE. Predetermined secondary endpoints included duration and severity of initial overt HE episode, transplant-free survival at 168 days post-TIPS, and incidence of cirrhosis-related complications. Scheduled follow-up occurred every 28 days to asses for overt HE and minimal HE using the Psychometric Hepatic Encephalopathy Score.
Data Analysis: Modified intention-to-treat analysis (defined as patients who did undergo scheduled TIPS) was performed for the primary and secondary endpoints. Safety analysis performed for any patient who received study medication.
Funding: French Public Health Ministry.
Results: From October 2013 through June 2016, 197 patients were randomized; 194 received at least 1 dose of study medication (safety analysis), and 186 had TIPS placed (modified ITT analysis for efficacy). Study patients were primarily male (77%), mean age of 60 years old, had alcohol-related liver disease (86%), and had intractable ascites as indication for TIPS (81%). Thirteen percent had prior overt HE episodes. For the primary endpoint, the incidence of overt HE was significantly lower in rifaximin-treated patients vs placebo-treated patients: 34% vs 53%, respectively, odds ratio (OR) 0.48; 95% confidence interval (CI): 0.27-0.87. (Figure 1) If isolated asterixis is not graded as overt HE, then rifaximin-treated patients still have lower incidence of overt HE: 20% vs 40%, respectively, P = 0.010. In post hoc analysis of 162 patients without prior overt HE episodes, the incidence of overt HE during 168 days follow-up trended lower in rifaximin-treated patients: 35% vs 51%, stratified log-rank P = 0.070. There were no significant differences in transplant-free survival, cirrhosis-related complications, incidence of minimal HE, or adverse events based on the safety analysis.
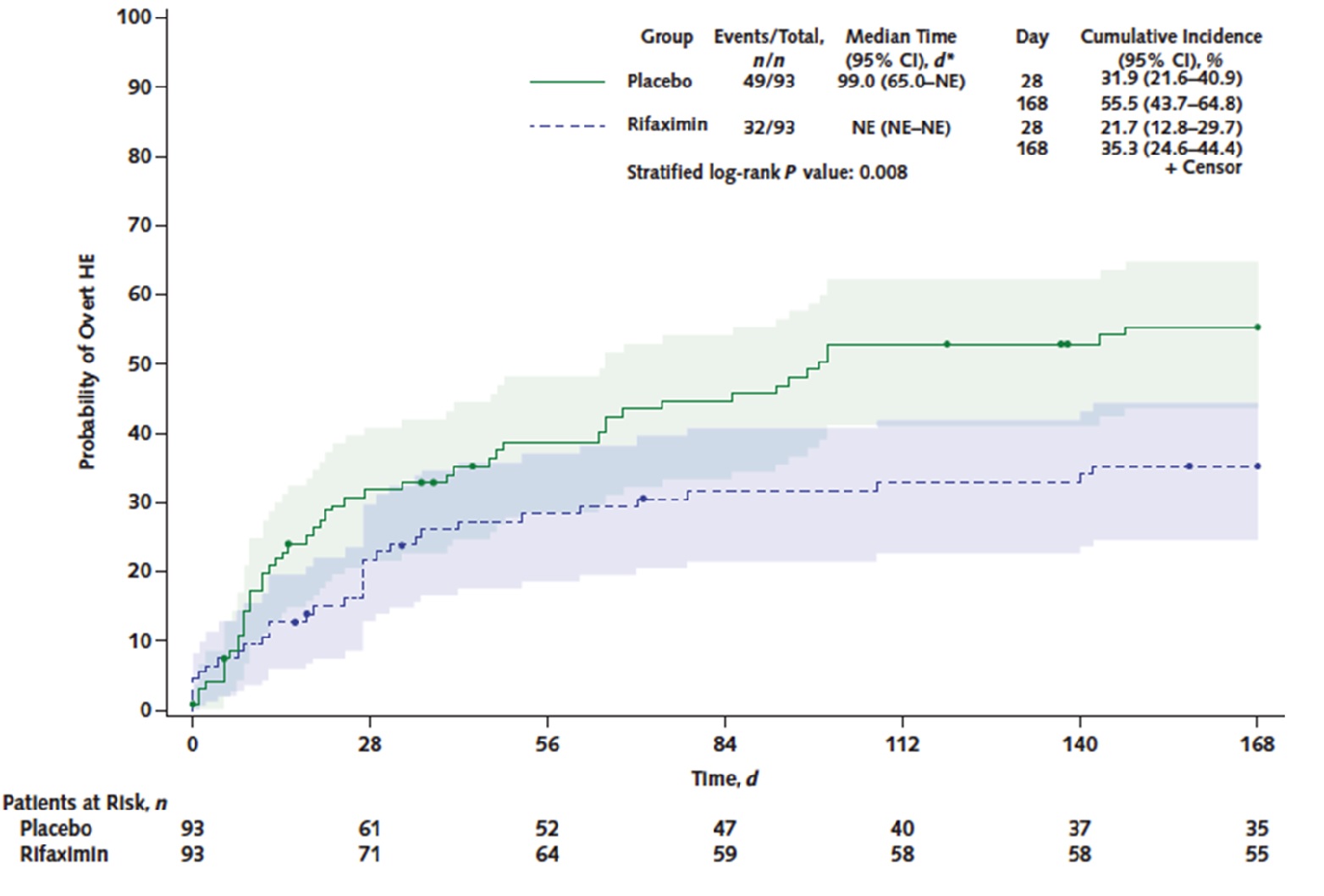
Figure 1. Incidence of overt hepatic encephalopathy procedures
COMMENTARY
Why Is This Important?
Since the introduction of covered stents for TIPS has reduced shunt dysfunction, the primary complication of TIPS is overt HE episodes, which occur in up to 50% of cirrhotic patients after TIPS and which usually lead to hospitalization. Although this is a frequent complication, prophylaxis against HE was not recommended in the 2014 American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) joint guideline on HE1, primarily due to a lack of data. At that time, only one RCT2 assessed this issue by randomizing 75 post-TIPS patients to rifaximin, lactulose, or no treatment and showed no difference in overt HE 1 month post-TIPS.
This well-designed RCT overcame these limitations by enrolling a larger sample size and following patients for almost 6 months post-TIPS. The investigators should be commended for their excellent study design and diligence to address this important issue.
For the primary endpoint, the cumulative incidence of overt HE was significantly lower in rifaximin-treated patients vs placebo-treated patients: 34% vs 53%, respectively, OR 0.48; 95% CI: 0.27-0.87.
Caution
Prophylactic use of lactulose use was not permitted, even among the 13% of patients who had a prior episode of overt HE, which is the standard of care in the US.
My Practice
Although this study demonstrated a significant reduction in cumulative incidence of overt HE, the most recent 2022 North American guidance on TIPS management3 does not recommend routine HE prophylaxis with rifaximin pending further RCT data. The authors expressed concern that lactulose was not provided to the 13% of patients with prior overt HE episodes.
As a general gastroenterologist, I relied on several transplant hepatology colleagues for guidance on this issue. They assess each patient for additional HE risk factors (advanced age, Child-Pugh score) and make an individualized decision about using rifaximin (or lactulose) as prophylaxis for overt HE. Although rifaximin is more convenient to use than lactulose, cost and lack of insurance coverage sometimes limit its use. Nevertheless, based on the current RCT, rifaximin has clearly demonstrated efficacy for this indication while RCT data for lactulose efficacy is lacking.
For Future Research
Additional confirmatory studies will be needed, including in the US, before guidelines routinely recommend rifaximin (or lactulose) treatment post-TIPS. It’s also unclear if HE prophylaxis should continue indefinitely after TIPS, especially since several study patients experienced overt HE shortly after discontinuing rifaximin at 168 days post-TIPS.
Conflict of Interest
Dr. Schoenfeld reports being an advisory board member and consultant for Salix Pharmaceuticals.
REFERENCES
- Vilstrup H, Amodio P, Bajaj J, et al. Hepatic Encephalopathy in Chronic Liver Disease: 2014 Practice Guideline by AASLD and EASL. Hepatology 2014; 60: 715-35.
- Riggio O, Masini A, Efrati C, et al. Pharmacological prophylaxis of hepatic encephalopathy after TIPS. J Hepatol 2005; 42: 674-79.
- Boike JR, Thornburg BG, Asrani SK, et al. North American Practice-Based Recommendations for Transjugular Intrahepatic Portosystemic Shunts in Portal Hypertension. Clin Gastroenterol Hepatol 2022; 20: 1636-62.

