Ozanimod for Moderate-Severe Ulcerative Colitis: Rethinking the Top-Down Treatment Algorithm
Oriana Damas, MD1 and Philip Schoenfeld, MD, MSEd, MSc (Epi)2

1Assistant Professor of Medicine, University of Miami School of Medicine, Miami, FL
2Chief (Emeritus), Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI.
This summary reviews Sandborn WJ, Feagan BG, D’Haens G, et al. Ozanimod for Induction and Maintenance of Ulcerative Colitis. N Engl J Med 2021; 385: 1280-91.
Correspondence to Philip Schoenfeld, MD, MSEd, MSc. Editor-in-Cheif. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Is ozanimod (Zeposia; Bristol Myers Squibb, Princeton, NJ), a selective sphingosine-1-phosphate receptor modulator, superior to placebo for induction and maintenance of remission in moderately to severely active ulcerative colitis (UC)?
Design: To assess induction of remission at 10 weeks, a multi-center, double-blind, placebo-controlled randomized controlled trial (RCT) was conducted, followed by a 42-week, multi-center, double-blind, placebo controlled RCT for UC patients with clinical response to assess maintenance of remission (True North study). Additionally, an additional cohort of moderate-severe UC patients received open-label ozanimod for 10 weeks in order to ensure an adequate sample size for the maintenance of remission RCT.
Setting: RCTs completed in 285 sites in 30 countries between May 2015 and June 2020.
Patients: In the induction of remission RCT, patients were: (a) 18-75 years old; (b) confirmed UC diagnosis > 90 days; (c) moderate-severe UC based on a total Mayo Score of 6-12 with endoscopic subscore of 2-3, rectal bleeding subscore > 1, and stool frequency subscore > 1⸸. Exclusion criteria included active or chronic infection, clinically significant cardiovascular condition, history of uveitis or macular edema, and prior history of failing to induce remission with > 2 biologic agents. For the maintenance of remission RCT, patients had to at least achieve clinical response, defined as reduction in total Mayo Score of > 3 points and > 30% from baseline or similar modification using 3-component Mayo Score. All study patients had to have positive IgG antibody for varicella-zoster virus or complete varicella-zoster vaccination.
Interventions/Exposure: In the induction of remission RCT, patients were randomized 2:1 to ozanimod 0.92 mg po qd vs placebo for 10 weeks. In the maintenance of remission RCT, UC patients who achieved clinical response were randomized 1:1 to ozanimod 0.92 mg or placebo through week 52. A 7-day dose escalation was used with ozanimod initiation to minimize risk of bradycardia: 0.23 mg on days 1-4, 0.46 mg on days 5-7 and 0.92 mg thereafter.
Outcome: The primary endpoint was clinical remission using a 3-component Mayo Score and defined as: rectal-bleeding subscore = 0; stool-frequency subscore < 1 with a decrease of at least 1 from baseline; and, an endoscopy subscore < 1. Key secondary endpoints assessed during induction of remission RCT were: (a) clinical response; (b) endoscopic improvement, defined as endoscopy subscore < 1 without friability; and, (c) mucosal healing, defined as endoscopic improvement plus histologic remission. ⸸⸸ In addition to standard safety analyses, pre-specified adverse events of interest were serious or opportunistic infection, cancer, bradycardia, heart block, macular edema, pulmonary and hepatic effects with pulmonary-function testing, ophthalmologic examination, electrocardiogram (ECG), leukocyte counts, and liver function tests (LFTs) performed before and during the trial.
Data Analysis: Modified intention-to-treat analysis defined as patients who were randomized and received at least 1 dose of study medication was performed for the primary endpoints with a 2-sided Cochran-Mantel-Haenszel test. The key secondary endpoints were assessed in a closed, prespecified hierarchical procedure. ⸸⸸ Safety analysis was performed for any patient who received study medication in both induction and maintenance RCTs.
Funding: Bristol Myers Squibb Pharmaceuticals, manufacturers of ozanimod.
Results: Six hundred forty-five patients were enrolled and included in efficacy analysis for the induction of remission RCT. Patient characteristics included male: 60%, mean age: 41-42, mean disease duration: 6.8 years, mean total Mayo Score at baseline = 8.9, and prior anti-TNF therapy = 30%. Clinical remission was significantly more common with ozanimod 0.92 mg po qd vs placebo for induction of remission (18.4% vs 6.0%, P < 0.001) and for all key secondary endpoints (Figure 1). For the maintenance of remission RCT, which included additional UC patients who achieved clinical response in an open-label cohort, 457 patients were randomized, and ozanimod was again superior to placebo for maintenance of remission: 37.0% vs 18.5%, P < 0.001.
Frequency of serious infections were similar in the ozanimod and placebo groups in the induction and maintenance RCTs and was < 2% in all groups. Absolute lymphocyte count decreased by a mean of 54% in the ozanimod-treated patients during induction of remission RCT. Elevated liver aminotransferase levels were more common with ozanimod vs placebo. Macular edema was reported in 3 patients, but this resolved after discontinuing therapy. No episodes of heart block were recorded. Although patients had to have varicella-zoster vaccination or IgG antibody, herpes zoster infection occurred in 2.2% of ozanimod-treated patients in the maintenance of remission RCT.
NOTES
⸸The Mayo Score assesses rectal bleeding score (0-3), stool frequency score (0-3), endoscopy sub score (0-3), and Physician’s Global Assessment (0-3), with a score range 0-12, with 12 representing most severe UC.
⸸⸸Although these trials used a classic double-blind, placebo-controlled, randomized study design with modified ITT analysis, study methodology and results are too detailed to summarize comprehensively. Readers are encouraged to review the full study publication.
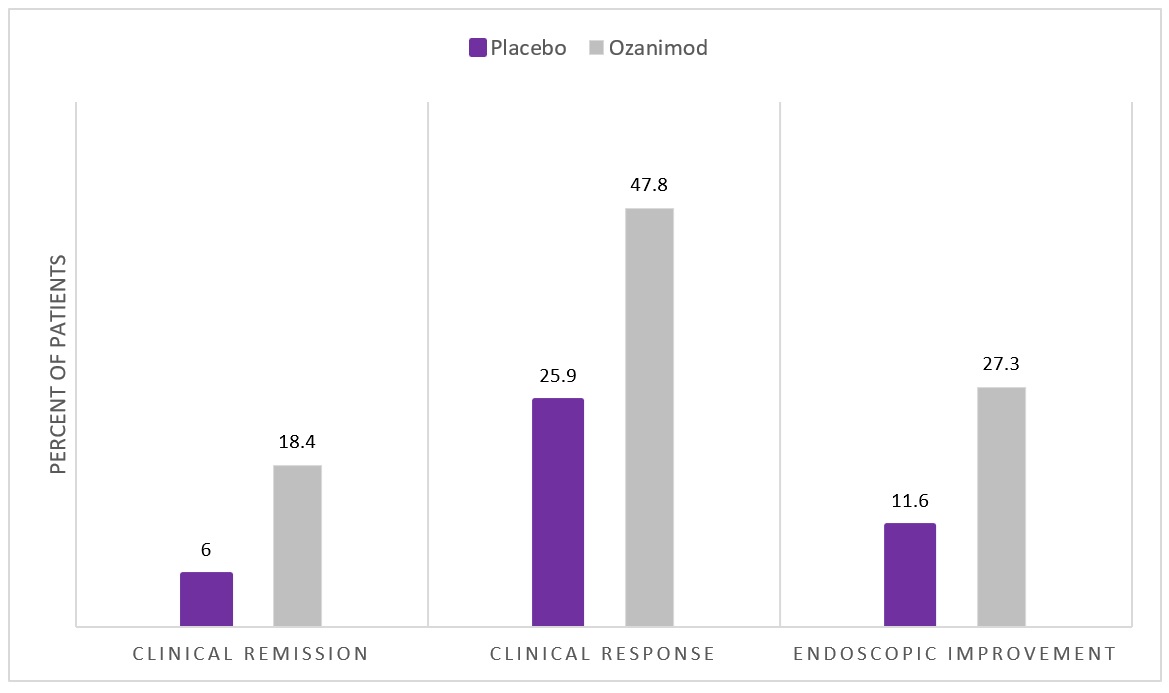
Clinical Remission: 3-component Mayo score with rectal-bleeding subscore = 0; stool-frequency subscore < 1 with a decrease of a least 1 from baseline; and endoscopy subscore < 1.
Clinical Response: reduction in total Mayo Score of > 3 points and >30% from baseline or similar modification using 3-component Mayo Score.
COMMENTARY
Why Is This Important?
As discussed in prior summaries1, multiple UC treatments have become available in the past 5 years. In addition to commonly used anti-TNF antibody treatments like infliximab (Remicade; Janssen Biotech, Horsham, PA) and adalimumab (Humira; AbbVie Biotechnology, Chicago, IL), anti-integrin antibody treatments like vedolizumab (Entyvio; Takaka Pharmaceuticals, Lexington, MA), anti-interleukin-12/23 antibodies such as ustekinumab (Stelara; Janssen Biotech), and selective JAK1 inhibitors like upadacitinib (Rinvoq; AbbVie Biotechnology) are FDA-approved for use. Given this expanding menu of therapies, new algorithms are needed to help gastroenterologists choose preferred treatment for individual UC patients by accounting for the strengths and limitations of individual agents.2
Although comparative RCTs are not available, upadacitinib, an oral selective JAK1 inhibitor with a relatively rapid onset of action, was superior for induction of remission to other biologics and small molecules in 2 recent network meta-analyses3-4. However, upadacitinib is approved for use only after inadequate response or intolerance to an anti-TNF agent.
Ozanimod is a selective sphingosine-1-phosphate receptor modulator, which leads to internalization of S1P1 receptors in lymphocytes and the prevention of lymphocyte mobilization to inflammatory sites and has also been used since 2020 for relapsing multiple sclerosis. Per the prescribing information, it’s contraindicated in patients with major adverse cardiac events in the past 6 months, presence of second or third degree heart block, and severe sleep apnea. Elevation of liver transaminases, bradycardia, decreased lymphocyte counts, and macular edema are also risks. Therefore, it is suggested that patients should have complete blood count, ECG, LFTs prior to initiating therapy. Patients should be vaccinated against varicella-zoster virus or demonstrate antibodies to the virus prior to initiating treatment. In order to minimize the risk of bradycardia, patients should complete a 7-day titration by using 0.23mg daily for day 1-4, 0.46 mg daily for days 5-7, followed by increasing to standard dose of 0.92 mg daily.
Ultimately, Sandborn and colleagues should be commended for designing a methodologically rigorous RCT and getting study patients through a rigorous study protocol. Given the morbidity and mortality associated with moderate-severe UC, the addition of ozanimod is welcome.
Key Study Findings
Caution
Ozanimond is contraindicated in patients with a recent history of major adverse cardiac events, history of heart block, or severe sleep apnea. LFTs and lymphocyte counts should be monitored, and the patient should be aware that it can increase the risk of macular edema, declines in pulmonary function, and herpes zoster infections despite vaccination.
My Practice
Our preferred use of ozanimod is for UC patients with moderate disease activity who prefer an oral agent and who do not have any of the risk factors for the above-mentioned contraindications. For example, we avoid ozanimod in UC patients with a history of uveitis. If patients are diabetic, then we routinely get an ophthalmologic exam before starting ozanimod. We avoid using it in patients with severe snoring, which may represent undiagnosed sleep apnea, and tend to avoid it in women of child-bearing age given the absence of data about its safety during pregnancy. Ultimately, we individualize our care by reviewing risks and benefits of different therapies with each patient and conduct shared decision making.
Prior to prescribing ozanimod, we follow our standard protocol of recommending vaccination against multiple infections, including herpes zoster. In addition to baseline laboratory assessment (CBC, comprehensive metabolic profile) and ECG, we check carefully to ensure that there are not pre-existing cardiac conditions, sleep apnea or other pulmonary disease, or symptoms of uveitis. As part of our nutrition assessment, we also caution patients to limit intake of tyramine-rich foods (e.g., aged cheeses) since ozanimod-treated patients are at higher risk of side effects like hypertension if they consume more than 150 mg of tyramine.
For Future Research
Ongoing RCTs will define efficacy of ozanimod for Crohn’s disease. Given the increasing number of available agents with different mechanisms of actions, comparative RCTs would be welcome to help establish positioning of therapies as well as longer-term safety data.
Conflict of Interest
Dr. Damas reports being an advisory board member for Janssen Pharmaceuticals, consultant for AbbVie Pharmaceuticals, and receiving research support from Pfizer Pharmaceuticals. Dr. Schoenfeld reports no conflicts of interest.
@silvio_silvio75
REFERENCES
- Kinnucan J, Schoenfeld P. Upadacitinib, a Selective JAK1 Inhibitor, for Moderate-Severe Ulcerative Colitis: Adjusting the Top-Down Treatment Algorithm for UC. Evidence-Based GI: An ACG Publication Oct 2022: 1-7. https://gi.org/wp-content/uploads/2022/10/Schoenfeld_October2022Final.pdf
- Baumgart DC, Le Berre C. Newer Biologic and Small-Molecule Therapies for Inflammatory Bowel Disease. N Engl J Med 2021; 385: 1302-15.
- Burr NE, Gracie DJ, Black CJ, Ford A. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut 2022; 71: 1976-87.
- Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2022; 7: 161-70
Download the article summary (PDF)

