Treating Helicobacter pylori Infection With Vonoprazan, A Potassium-Competitive Acid Blocker: A New Paradigm

Philip Schoenfeld, MD, MSEd, MScEpi, FACG
Chief Emeritus-Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, Michigan
This summary reviews: Chey WD, Megraud F, Laine L, et al. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the US and Europe: A Randomized Controlled Trial. Gastroenterology 2022 Jun 6;S0016-5085(22)00609-6. DOI: 10.1053/j.gastro.2022.05.055
Access the Article through PubMed
Correspondence to Dr. Philip Schoenfeld, Editor-in-Chief. Email: EBGI@gi.org
STRUCTURED ABSTRACTQuestion: Are vonoprazan triple (Voquezna TriplePak) and dual regimens (Voquezna DualPak) non-inferior to standard lansoprazole-based triple regimen (Prevpac) for treatment-naïve individuals with Helicobacter pylori infection?
Design: Phase III, multicenter, double-blind randomized controlled trial.
Setting: Patients from 103 sites in the US, United Kingdom, Bulgaria, the Czech Republic, Hungary, and Poland.
Patients: Included patients were: (a)> 18 years old; (b) indication to test for H. pylori, including dyspepsia, recent diagnosis of non-bleeding peptic ulcer, history of peptic ulcer with no prior treatment of H. pylori, or requirement for long-term NSAID use; (c) positive 13C-urea breath test for H. pylori infection; and (d) no prior treatment for H. pylori infection. All eligible patients then underwent eophagogastroduodenoscopy with biopsy for culture and antimicrobial susceptibility testing as well as histology. All study patients had active H. pylori infection confirmed by culture or histology.
Interventions/Exposure: Eligible patients were randomized 1:1:1 to open-label vonoprazan dual therapy (vonoprazan 20 mg b.i.d. plus amoxicillin 1g t.i.d X 14 days) vs double-blind vonoprazan triple therapy (vonoprazan 20 mg b.i.d. plus amoxicillin 1 g b.i.d. plus clarithromycin 500 mg b.i.d. X 14 days) vs lansoprazole triple therapy (lansoprazole 30 mg b.i.d. plus amoxicillin 1 gm b.i.d. plus clarithromycin 500 mg b.i.d. X 14 days).
Outcome: The primary endpoint was H. pylori eradication based on negative 13C-urea breath test obtained at least 4 weeks after last dose of study medication. Patients with persistent H. pylori infection underwent repeat eophagogastroduodenoscopy with repeat antimicrobial susceptibility testing. Per FDA guidance, the primary non-inferiority endpoint was assessed in the study patients with H. pylori strains that were not resistant to clarithromycin or amoxicillin. Pre-determined secondary endpoints assessed frequency of H. pylori eradication in all study patients and frequency of eradication in study patients with clarithromycin-resistant strains of H. pylori.
Data Analysis: Modified intention-to-treat analysis and per-protocol analysis (defined as patients who took > 75% of study drug) was performed for the primary endpoint and both secondary endpoints. Analyses were conducted in a hierarchical order for each comparison: vonoprazan dual therapy vs lansoprazole triple therapy and vonoprazan triple therapy vs lansoprazole triple therapy for non-inferiority of H. pylori eradication among patients with strains that were susceptible to clarithromycin and amoxicillin. Again, this analysis was guided by the US Food and Drug Administration (FDA). Secondary endpoints were then assessed using superiority analysis for clarithromycin-resistant strains and for all patients.
Funding: Phathom Pharmaceuticals, manufacturer of vonoprazan.
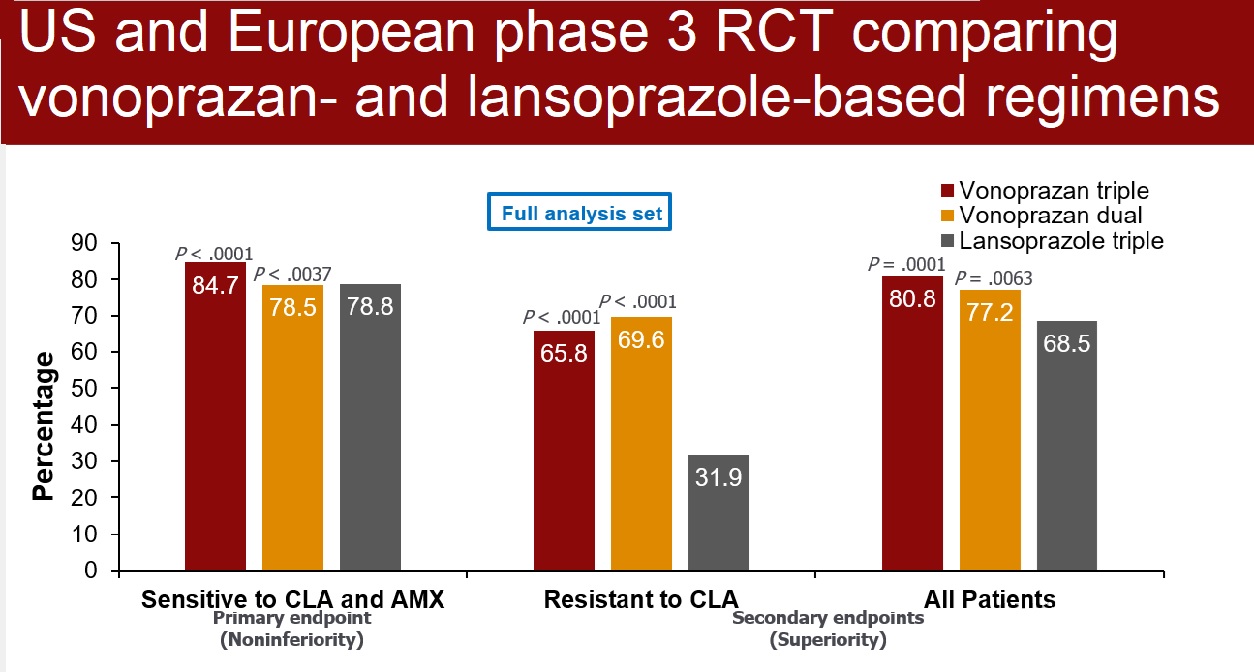
Results: From December 2019 through January 2021, 3,385 patients were screened for eligibility, 1,046 were randomized, and 992 were fully evaluated (mean age: 51-52 years old; 37% male; 90% White; 42% from US; 98% with dyspepsia as indication to test for H. pylori; 20% with clarithromycin-resistant strains; 1% with amoxicillin-resistant strains; 63% with metronidazole-resistant strains). For the primary endpoint requested by the FDA, vonoprazan dual therapy and vonoprazan triple therapy were non-inferior to lansoprazole triple therapy for eradication of H. pylori with no resistance to clarithromycin or amoxicillin (78.5% vs 84.7% vs 78.8%, respectively) (Figure 1). Vonoprazan dual therapy and vonoprazan triple therapy were superior to lansoprazole triple therapy for H. pylori eradication when evaluating all patients (77.2% vs 80.8% vs 68.5%, respectively, P < 0.01) and when evaluating patients with clarithromycin-resistant strains (69.6% vs 65.8% vs 31.9%, respectively, P < 0.001). (Figure 1)
Figure 1: Helicobacter pylori eradication rates.
AMX, amoxicillin ; CLA, clarithromycin; RCT, randomized controlled trial.
COMMENTARY
Why Is This Important?
Quite simply, clansoprazole-based triple therapy with clarithromycin should not be used unless antimicrobial sensitivity testing has confirmed clarithromycin-sensitivity.1 This is because lansoprazole-based triple therapy with clarithromycin and similar regimens only achieve 30% eradication rates in clarithromycin-resistant strains, and rates of clarithromycin resistance exceed 20% in most parts of the US. As seen in this study, this translates to successful eradication in only about 65%-70% of US patients with lansoprazole-based triple therapy with clarithromycin, which is far from optimal. Nevertheless, US database studies estimate that lansoprazole-based triple therapy with clarithromycin and similar regimens account for almost 50% of prescriptions.
Bismuth-based quadruple therapy and rifabutin-based triple therapy (Talicia) for 14 days are preferred regimens.1,2 Unfortunately, compliance and pill burden make bismuth-based quadruple therapy regimens cumbersome and use of rifabutin-based therapy seems to have been limited by cost, limited formulary availability, co-pays, and lack of awareness. Therefore, the recent FDA approval3 of vonoprazan-based dual therapy and vonoprazan-based triple therapy for H. pylori treatment are welcome.
Vonaprazan, a potassium-competitive acid blocker, produces earlier and more potent acid suppression than conventional proton pump inhibitors and this translates to more successful treatment of H. pylori. Specifically, enhanced gastric acid suppression optimizes the activity of amoxicillin and clarithromycin, which may be destabilized by lower pH. Also, active bacterial replication of H. pylori increases at higher gastric pH and this replication facilitates antibiotic eradication.
Key Study Findings
Vonoprazan dual therapy and vonoprazan triple therapy were superior to lansoprazole triple therapy for H. pylori eradication when evaluating all patients (77.2% vs 80.8% vs 68.5%, respectively, P < 0.01) and when evaluating patients with clarithromycin-resistant strains (69.6% vs 65.8% vs 31.9%, respectively, P < 0.001) (Figure 1).
Caution
Since only treatment-naïve H. pylori patients were enrolled, this study doesn’t provide data about management of patients with refractory H. pylori infections. Otherwise, this is a very well-designed, Phase III RCT, which met all FDA requirements and led to FDA approval of vonoprazan-based dual therapy and triple therapy regimens.
My Practice
At my VA Medical Center, my current preferred therapy is bismuth-based quadruple therapy and I’m limited by my formulary to only using rifabutin-based triple therapy for salvage therapy in patients with refractory H. pylori infection.
Vonoprazan-based dual therapy with amoxicillin is ideal for good antibiotic stewardship.4 If cost and availability concerns can be addressed at my VA Medical Center, then this will be my preferred first-line therapy.
It’s also worth emphasizing the ACG Guideline recommendation that post-treatment testing should be performed routinely to ensure successful eradication.2 I prefer to do this with stool antigens for H. pylori and have the specimen collected at least 4 weeks after completing antibiotics and 2 weeks after discontinuing acid suppression therapy.
For Future Research
Future studies should assess real-world efficacy of vonoprazan-based therapies in patients with refractory H. pylori infection. Additional studies should use next-generation sequencing5 or other tools to better define rates of antibiotic-resistant strains in different geographic regions of the US.5 Quality improvement research should also assess interventions to improve frequency of testing for eradication 4 weeks after patients complete treatment.
Conflict of Interest
Dr. Schoenfeld reports being an advisory board member and consultant for Phathom Pharmaceuticals.
REFERENCES
- Howden C, Graham DY. Recent Developments Pertaining to H. pylori Infection. Am J Gastroenterol 2021; 116: 1-3.
- Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol 2017; 112: 212-38.
- Voquezna Prescribing Information. Phathom Pharmaceuticals. Accessed at www.accessdata.fda.gov on May 10, 2022.
- Graham DY, Liou JM. Primer for Development of Guidelines for Helicobacter pylori Therapy Using Antimicrobial Stewardship. CGH 2022; 20: 973-83.
- Graham DY, Moss SF. Antimicrobial Susceptibility Testing for Helicobacter Pylori Is Now Widely Available: When, How, Why. Am J Gastroenterol 2022; 117: 524-28.