Dupilumab, an IgG4 Monoclonal Antibody, for Eosinophilic Esophagitis: Revising the Treatment Paradigm
Afrin Kamal, MD, MS1 and Philip Schoenfeld, MD, MSEd, MSc (Epi)2
1Clinical Assistant Professor of Medicine, Stanford University School of Medicine, Palo Alto, CA
2Chief (Emeritus), Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI.
This summary reviews Dellon ES, Rothenberg MH, Collins I, et al. Dupilumab in Adults and Adolescents with Eosinophilic Esophagitis. N Engl J Med 2022; 387: 2317-30.
Access the article through PubMed
Correspondence to Philip Schoenfeld, MD, MSEd, MSc. Editor-in-Chief. Email: EBGI@gi.org
STRUCTURED ABSTRACT
Question: Are weekly subcutaneous injections of dupilumab (Dupixent; Regeneron Pharmaceuticals, Tarrytown, NY), an anti-interleukin-4/13 monoclonal antibody, superior to placebo for inducing histologic remission and symptomatic improvement in swallowing for eosinophilic esophagitis (EoE) in adults and adolescents (>12 to <18 years old)?
Design: To assess induction of remission at 24 weeks, 2 multi-center, double-blind, placebo-controlled randomized controlled trials (RCTs) were conducted (Part A and Part B). A single multi-center, active treatment extension study through week 52 was also performed (Part C). Randomization was stratified for age (adolescent [>12 years and < 18 years old] vs adults [>18 years old]) and current use of proton pump inhibitors (PPIs).
Setting: Ninety-six centers across Australia, Canada, Europe, and the United States, with the US accounting for the majority (n = 63) of sites.
Patients: Eligible patients were: (a) >12 years old; (b) had a confirmed EoE diagnosis based on >15 eosinophils per high power field (hpf) after 8 weeks of high-dose PPI therapy; and, (c) >10 on Dysphagia Symptom Questionnaire (DSQ; range 0-84 with higher scores identifying more severe and/or more frequent dys-hagia). Study patients were allowed to continue on stable doses of PPI and/or stable food elimination diets at study entry, but could not start PPI or food elimination diets after study entry.
Interventions/Exposure: In Part A, patients were randomized 1:1 to dupilumab 300 mg subcutaneous (subq) weekly vs placebo subq weekly for 24 weeks. In Part B, patients were randomized 1:1:1 to dupilumab 300 mg subq weekly vs dupilumab 300 mg subq every 2 weeks vs placebo subq every 2 weeks. In Part C, where data is only available from patients who participated in Part A, all patients were treated with dupilumab 300 mg subq weekly for an additional 28 weeks (52 weeks total), regardless of whether they originally received dupilumab or placebo during Part A.
Outcome: Co-primary endpoints were histologic remission, defined as <6 eosinophils per hpf) and change from baseline in DSQ. Multiple secondary endpoints were also evaluated, including absolute change from baseline in the endoscopic reference scoring system, EREFS, which stands for edema, rings, exudates, furrows, and strictures (range 0-18 with higher scores indicating more severe endoscopic findings).
Data Analysis: Histologic remission and binary secondary endpoints were analyzed with Cochran-Mantel-Haenszel test, and absolute changes in the DSQ and continuous secondary endpoints were analyzed with analysis of covariance. Safety analysis was performed for any patient who received at least 1 dose of study medication (dupilumab or placebo).
Funding: Sanofi and Regeneron Pharmaceuticals, manufacturers of dupilumab.
Results: Patients in Part A (n=81) and Part B (n=240) had EoE for mean of 5-5.6 years with mean peak eosinophil count of 87-89, mean DSQ score of 33-37, mean EREFS score of 6.3-7.2, and 36%-41% were on food elimination diet at screening. Histologic remission was significantly more common with dupilumab 300 mg subq weekly vs placebo in Part A (60% vs 5%) and Part B (59% vs 6%) (Table 1). Dupilumab 300 mg subq weekly also produced significantly larger reductions from baseline in dysphagia symptoms per DSQ in Part A (68% vs 27%) and Part B (62% vs 38.5%). Although histologic remission was significantly more common with dupilumab 300 mg subq every 2 weeks vs placebo in Part B, it did not produce a statistically significant decrease in DSQ scores vs placebo. Reduction in EREFS score was also greater with dupilumab 300 mg subq weekly vs placebo in Part A (-3.2 vs -0.3) and Part B (-4.5 vs -0.6). In Part C, where all patients from Part A received dupilumab 300 mg subq weekly for an additional 28 weeks, histologic remission and reduction in DSQ was sustained among patients who originally received dupilumab and similar rates of histologic remission and reduction in DSQ were observed in patients switched from placebo to dupilumab. No significant differences in adverse events occurred.
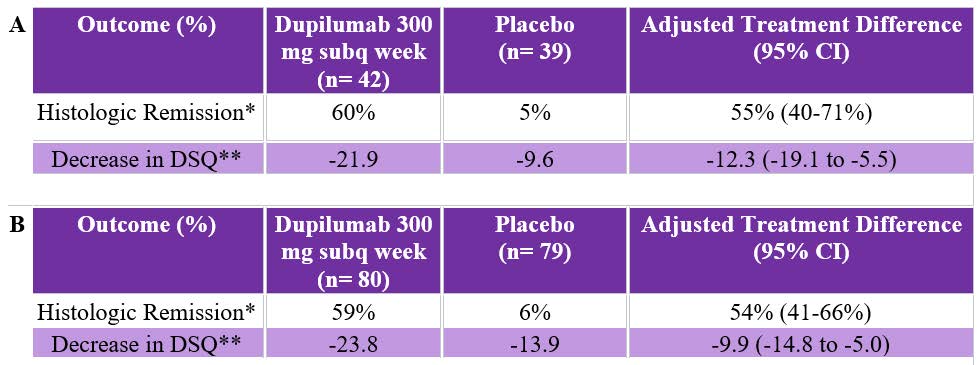 Table 1. Co-Primary Endpoint Outcomes of Part A and Part B Randomized Controlled trials.
Table 1. Co-Primary Endpoint Outcomes of Part A and Part B Randomized Controlled trials.*Histologic Remission: < 6 eosinophils per high power field
**Decrease in DSQ: Absolute numeric decrease in score on DSQ, which has a range of 0-84 with higher scores identify-ing more severe or more frequent dysphagia. In Part A, mean dysphagia score at baseline = 33.6 and was 36.7 at baseline in Part B.
CI, confidence interval ; DSQ, Dysphagia Symptom Questionnaire; subq, subcuteanous.
COMMENTARY
Why Is This Important?
The incidence and prevalence of EoE, an immune-mediated, chronic allergic inflammatory condition of the esophagus, has steadily risen as endoscopists began to routinely obtain biopsies from patients who presented with the characteristic symptoms of dysphagia or food impaction.1-2 Although PPIs, swallowing corticosteroids from metered dose inhalers approved for asthma (e.g., Flovent, Pulmicort), and 6-food elimination diets (dairy, wheat, soy, eggs, nuts, and shellfish) were the mainstays of therapy, their efficacy is limited and marred by poor adherence.3-5 Unfortunately, it’s unlikely that oral suspensions of budesonide will become available in the US in the near future since Takeda Pharmaceuticals discontinued its development program in 2022. Given these limitations, the introduction of dupilumab 300 mg subq weekly as the first FDA-approved treatment for EoE is a very welcome event.
Dupilumab is a monoclonal antibody that binds to the interleukin-4 receptor α-subunit, which is shared by the cytokine IL-4 and IL-13 receptors. This inhibits the type 2 helper T-cell inflammation that involves T cells, mast cells, cytokines IL-4, IL-13, and IL-5, and eosinophils, which is associated with asthma, atopic dermatitis/eczema, chronic rhinosinusitis as well as EoE. This Phase 3 RCT clearly demonstrates its efficacy for clinically important dysphagia symptoms and histologic remission, which is important since symptomatic improvement and histologic remission sometimes do not correlate. Importantly, these data also demonstrate improvement in the structural damage (e.g., rings, strictures) due to EoE inflammation based on the EREFS score.
Safety is very important with any new class of drugs. Although only approved for EoE since summer 2022, dupilumab has been FDA-approved for various eosinophilic-mediated inflammatory disorders since 2017, has extended safety data in these disorders, and is actually approved for use in pediatric patients as young as 6 months old with atopic dermatitis/eczema. Although eosinophil counts decrease with dupilumab use, no monitoring of complete blood cell counts, comprehensive metabolic profiles/liver function tests, or screening for opportunistic infections are recommended with dupilumab.
Histologic remission was significantly more common with dupilumab 300 mg subq weekly vs placebo in Part A (60 vs 5%) and Part B (59% vs 6%) (Table 1). Dupilumab 300 mg subq weekly also produced significantly larger reductions from baseline in dysphagia symptoms per DSQ in Part A (68% vs 27%) and Part B (62% vs 38.5%).
Caution
Study patients had already tried PPIs and failed to get histologic remission or
adequate relief of dysphagia, which is similar to other studies of EoE treatments. Approximately 40% had used or were using food elimination diets at the onset of trial, too. Therefore, the efficacy of dupilumab in treatment-naïve patients is unclear. Dupilumab is significantly more expensive than PPIs, food elimination diets or swallowing the content of corticosteroid metered dose inhalers. Although the 52-week data reported in this trial is longer than virtually any other trial, it’s still a relatively short period of follow-up for a chronic, lifelong condition.
My Practice:
First, we adhere to recently published quality indicators for the diagnosis and management of EoE.2 Specifically, we obtain at least 6 biopsies from at least 2 esophageal levels in patients with dysphagia without a known etiology, and we also obtain these biopsies when endoscopically treating food impactions as long as it is medically safe. Approximately 3-4 months after initiating a specific therapy or changing therapies, we will order a repeat EGD to assess for histologic improvement since symptomatic response may not correlate with histologic remission. We also educate that this is a chronic condition and that maintenance therapy is needed even after clinicopathologic remission in order to minimize the risk of recurrent symptoms and fibrosis/stricturing in the esophagus.
Our standard approach is to educate patients about the risks and benefits of PPIs, food-elimination diets, swallowed corticosteroids from a metered dose inhaler, and dupilumab. Generally, we recommend PPIs twice daily as initial therapy. If this is unsuccessful, then we utilize shared decision-making to choose between the other options. When using food-elimination diets, we start with 2-food elimination: dairy and wheat. One of us (AK) has had success with her patient population in Northern California, although it’s been difficult for the other co-author (PS) to implement this in his midwestern Veteran population. Efficacy of topical corticosteroids may be facilitated with detailed instructions about how to swallow the contents of the inhaler, but adherence and clinicopathologic remission is variable. As the first FDA-approved treatment, dupilumab meets a clinically important need, especially in patients who have already failed alternative treatments, as long as insurance covers this medication and the patient commits to being adherent with weekly subq injections.
For Future Research
Longer-term data about efficacy, safety, and adherence with dupilumab in management of EoE patients would be welcome as well as data that identifies EoE patients that are most likely to achieve histologic remission and improvement in dysphagia with dupilumab. Cost-effectiveness and comparative RCTs of EoE treatments are also needed.
Conflict of Interest
Dr. Kamal reports serving as an advisory board member for Castle Biosciences. Dr. Schoenfeld reports serving as an advisory board member for Sanofi Pharmaceuticals.
@EvanDellon
@IkuoHirano
REFERENCES
- Staumann A. Biologics in Eosinophilic Esophagitis-Ready for Prime Time? N Engl J Med 2022; 387: 2379-80.
- Leiman DA, Kamal AN, Otaki F, et al. Quality Indicators for the Diagnosis and Management of Eosinophilic Esophagitis. Am J Gastroenterol 2023; In Press.
- Hirano I, Chan ES, Rank MA, et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020; 158: 1776-86.
- Bredenoord AJ, Patel K, Schoepfer AM, et al. Disease Burden and Unmet Need in Eosinophilic Esophagitis. Am J Gastroenterol 2022; 117: 1231-41.
- Haasnoot ML, Safi S, Bredenoord AJ. Poor Adherence to Medical and Dietary Treatments in Adult Patients with Eosinophilic Esophagitis. Am J Gastroenterol 2022; 117: 1412-18.
Download the Article Summary (PDF)



