Vonoprazan, a Potassium-Competitive Acid Blocker, Is Superior to Lansoprazole for Managing Erosive Esophagitis

Philip Schoenfeld, MD, MSEd, MSc (Epi)
Chief (Emeritus), Gastroenterology Section, John D. Dingell VA Medical Center, Detroit, MI
This summary reviews Laine L, DeVault K, Katz P, et al. Vonoprazan versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial. Gastroenterology 2022; In Press. doi: https://doi.org/10.1053/j.gastro.2022.09.041
Correspondence to Philip Schoenfeld, MD, MSEd, MSc (Epi), Editor-in-Cheif. Email: EBGI@gi.org
Access the article through PubMed
STRUCTURED ABSTRACT
Question: Is vonoprazan, a potassium-channel acid blocker (PCAB), non-inferior to lansoprazole for healing and maintenance of healing of erosive esophagitis?
Design: A phase III, multi-center, double-blind randomized controlled trial (RCT).
Setting: One hundred eleven sites in the US, United Kingdom, Bulgaria, the Czech Republic, Hungary, and Poland.
Patients: Included patients were > 18 years old and had erosive esophagitis on endoscopy, which was confirmed by blinded central reading of endoscopic images. Exclusion criteria included active Helicobacter pylori infection and Barrett’s esophagus.
Interventions/Exposure: Eligible patients were randomized 1:1 to vonoprazan 20 mg daily vs lansoprazole 30 mg daily for 8 weeks. Endos-copy to assess healing was performed at 2 weeks and 8 weeks. Study medication was taken 30 minutes before breakfast. Patients who achieved healing were re-randomized 1:1:1 to vonoprazan 20 mg daily, vonoprazan 10mg daily, or lansoprazole 15 mg daily X 24 weeks, followed by repeat upper endoscopy.
Outcome: The primary endpoint was healing of erosive esophagitis after 8 weeks of treatment for the initial treatment phase. Among patients who entered the maintenance phase, the primary endpoint was absence of ero-sive esophagitis at 24 weeks. Pre-determined sub-group analysis of patients with Los Angeles (LA) Grade C/D esophagitis at baseline was performed for both primary endpoints. During the initial 8-week treatment period, percentage of 24-hour heartburn-free days and proportion of subjects with onset of sustained heartburn resolution by day 3 were also assessed.
Data Analysis: Modified intention-to-treat analysis and per-protocol analysis (defined as patients who were compliant with study treatment, did not take additional PPI or H2 receptor antagonists, and completed all study endoscopies) was performed for the primary and secondary endpoints. Analyses were conducted in a hierarchical order, non-inferiority analyses with 10% margin were first performed for both healing and maintenance. If non-inferiority was demonstrated, then superiority analyses were performed.
Funding: Phathom Pharmaceuticals, manufacturer of vonoprazan.
Results: From November 2019 through December 2019, 1,027 patients were enrolled and randomized for the initial treatment phase (mean age: 51-52 years old; 38% male; 91% White; 63% from US; 34% LA Grade C/D esophagitis); 893 achieved healing and were randomized for the maintenance phase. Non-inferiority was demonstrated for defined primary and secondary endpoints. Vonoprazan 20 mg daily was superior to lansoprazole 30 mg daily for healing of erosive esophagitis (93% vs 85%, P< 0.0001) and for the sub-group of patients with LA Grade C/D esophagitis (92% vs 72%, P< 0.001) (Figure 1). Similarly, vonoprazan 20 mg daily and vonoprazan 10 mg daily was superior to lansoprazole 15 mg daily for maintenance of healing (81% vs 79% vs 72%, P< 0.0001) and in the sub-group of patients who initially had LA Grade C/D esophagitis (77% vs 75% vs 61%, P < 0.001). In the maintenance phase, higher pro-portions of vonoprazan-treated patients (20 mg and 10 mg daily) had heartburn-free days than with lansoprazole 15 mg daily: median 95.2% vs 94.6% vs 89.3%, P < 0.03. No significant difference in adverse events between groups were identified.
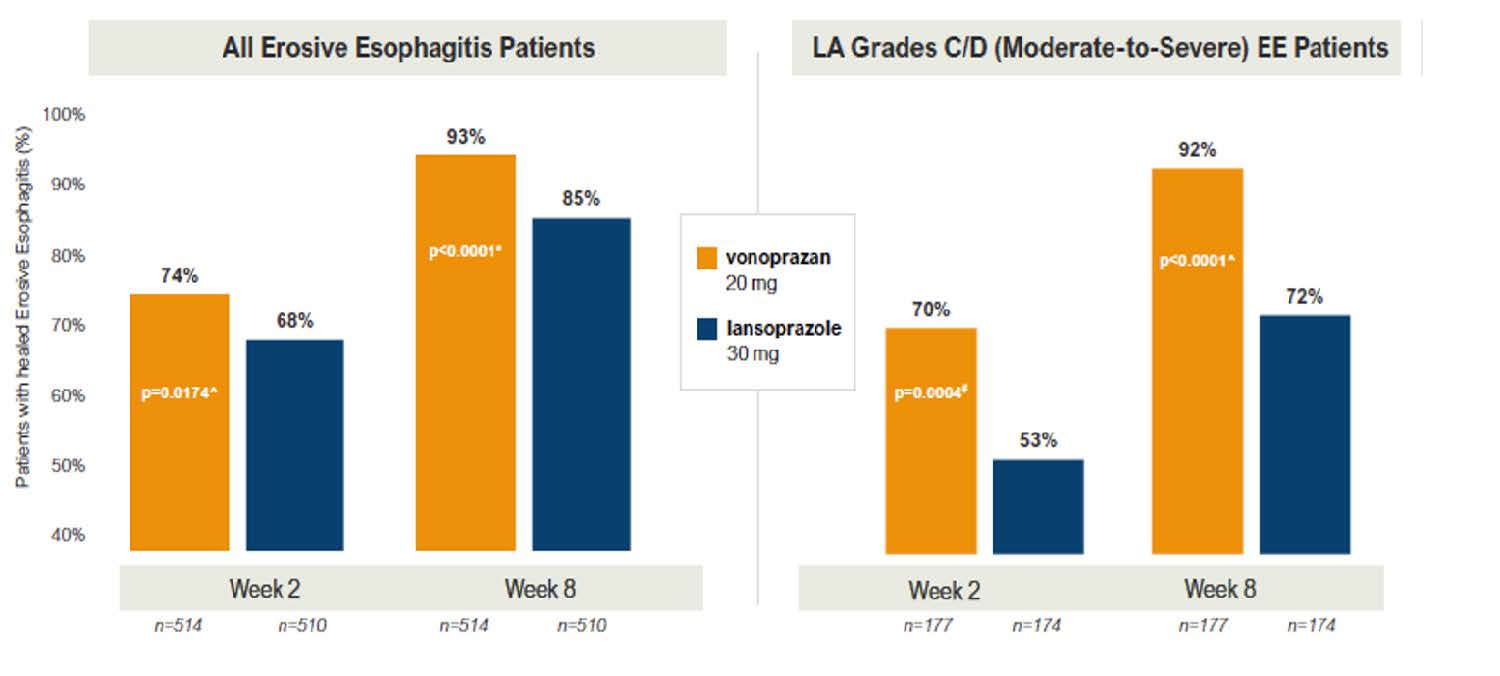
Figure 1: Healing of erosive esophagitis.
LA, Los Angeles
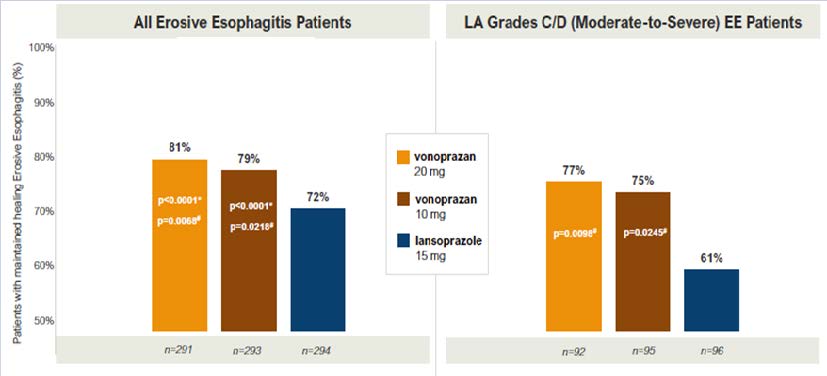
Figure 1: Maintenance of healing erosive esophagitis.
EE, erosive esophagitis
COMMENTARY
Why Is This Important?
Vonoprazan, a potassium-channel acid blocker, offers multiple pharmacologic advantages over proton pump inhibitors (PPIs).1 Since they are acid stable, they don’t need the enteric coating that acid labile PPIs require. This equates to a more rapid onset of action, which is sustained with a t1/2 of about 9 hours as opposed to the relatively short t1/2 of 1-2 hours for PPIs. PPIs bind only to activated proton pumps, which is why optimal dosing is 30 minutes before meals, while vonoprazan reversibly binds to the H, K-ATPase to compete with potassium binding, eliminating the need for pre-prandial dosing. Ultimately, PCABs produce more potent and more prolonged acid suppression with a more rapid onset compared to PPIs. Nevertheless, PPIs are still quite potent, and RCTs are needed to determine if PCABs are superior to PPIs for clinically important outcomes, including management of erosive esophagitis and heartburn.
Vonoprazan 20 mg daily was superior to lansoprazole 30 mg daily for healing of erosive esophagitis (93% vs 85%, P< 0.0001) and for the sub-group of patients with LA Grade C/D esophagitis (92% vs 72%, P < 0.001) (Figure 1). Similarly, vonoprazan 20 mg daily and vonoprazan 10 mg daily was superior tolansoprazole 15 mg daily for maintenance of healing (81% vs 79% vs 72%, P < 0.0001) and in the sub-group of patients who initially had LA Grade C/D esophagitis (77% vs 75% vs 61%, P< 0.001).
Caution
There were no significant differences in adverse events between vonoprazan and lansoprazole in this RCT. Unfortunately, the lay media has publicized retrospective case-control studies that suggest an association between the acid inhibition of PPIs and many different disorders. Similar concerns could be expressed about PCABs, which produce more potent acid inhibition. However, careful review of well-designed epidemiologic studies2 and placebo-controlled RCTs only demonstrate an increased risk of enteric infection with PPIs, but do not find PPIs associated with other disorders like dementia or fractures. It’s possible, but unproven, that PPIs could produce interstitial nephritis leading to decreased renal function. Even if this hypothesis is proven, this would not equate to a similar risk with PCABs. Nevertheless, US safety data is limited to approximately 34 weeks of use and longer-term safety data would be beneficial.
My Practice
Vonoprazan will not be commercially available in the US until 2023. When it is available, it will be my preferred treatment for healing and maintenance of LA Grade C/D erosive esophagitis as well as for PPI-resistant heartburn patients with confirmed abnormal esophageal acid exposure while on twice daily PPIs. It may also prove to be helpful for patients with breakthrough nocturnal heartburn despite PPI therapy combined with nightly H2RAs.
For Future Research
Vonoprazan has been available in Japan for several years. Additional safety data from Japanese databases would be welcome. Since vonoprazan has a rapid onset of action, it may be an option for on-demand treatment of heartburn. Finally, additional RCTs comparing vonoprazan with PPIs for improvement in the gastroesophageal reflux disease (GERD)-Health Related Quality of Life Questionnaire and other GERD symptoms would be helpful.
Conflict of Interest
Dr. Schoenfeld reports being an advisory board member and consultant for Phathom Pharmaceuticals.
@LorenLaine2
@katzpo
REFERENCES
- Katzka DA, Kahrilas P. PCAB Suppression of Gastric Acid in Erosive Esophagitis: Is Stronger and Longer Better? Gastroenterology 2022; In Press. doi: https://doi.org/101053/j.gastro.2022.10.022.
- Vajravelu R. Reinvestigating the Lack of Association Between Proton Pump Inhibitor Use and Mortality by Accounting for Reverse Causation. Evidence-Based GI; Nov 2022.
Download the article summary (PDF)

