In Case You Missed It
How Frequently Should We Perform Colonoscopy in Patients With Serrated Polyposis Syndrome?
 Timothy Yen, MD
Timothy Yen, MD
Assistant Professor of Medicine, Division of Gastroenterology, Loma Linda University School of Medicine, Loma Linda, CA
This summary reviews Bleijenberg AG, IJspeert JE, van Herwaarden YJ et al. Personalised surveillance for serrated polyposis syndrome: results from a prospective 5-year international cohort study. Gut 2020;69(1):112-121.
Access the article through PubMed
Correspondence to Timothy Yen, MD. Associate Editor. Email: EBGI@gi.org
Keywords: colonoscopy, serrated polyposis syndrome, polyp, colorectal cancer, surveillance
STRUCTURED ABSTRACT
Question: Among patients with serrated polyposis syndrome (SPS), can we increase colonoscopy intervals from annually to every 2 years based upon patient specific factors?
Study Design: Prospective cohort.
Setting: Nine international centers from Spain (33%) and Netherlands (66%).
Participants: All patients fulfilling 2010 World Health Organization (WHO) SPS criteria I and/or III1 who underwent endoscopic surveillance between January 2013 and April 2018 after initial polyp clearing colonoscopies (removal of all polyps ≥5 mm and those with an optical adenomatous or serrated appearance). Surveillance was started either before or during study period. Those with proctocolectomy, subtotal colectomy, inflammatory bowel disease or known colorectal cancer (CRC)-related germline genetic variants were excluded.
2010 WHO SPS type I criteria was defined as ≥5 serrated lesions/polyps (SSLs) proximal to the sigmoid colon, with at least 2 lesions/polyps ≥10 mm in size and type III was defined as ≥20 SSLs of any size distributed throughout the colon. Serrated polyps were defined as hyperplastic polyp ≥5 mm, SSL with or without dysplasia, or traditional serrated adenoma. Advanced adenomas were defined as adenomas ≥10 mm, with villous structure and/or with high-grade dysplasia (HGD). Advanced SSLs were defined as traditional serrated adenoma, any SSL ≥10 mm and/or with presence of dysplasia. Advanced neoplasia (AN) was defined as CRC, advanced adenoma, or advanced SSL.
Intervention: Annual surveillance colonoscopy was performed in those with ≥1 advanced adenoma/SSL, ≥5 adenomas/SSLs or if colorectal surgery was performed. All other SPS patients received surveillance colonoscopy every 2 years. Surveillance colonoscopies were done with the goal of removing of all polyps ≥5 mm and those with an optical adenomatous or serrated appearance.
Outcomes: Primary outcome was 5-year cumulative incidence of CRC and AN. Secondary outcomes were frequency of 1- vs 2-year surveillance interval recommendation, incidence of colorectal surgery during surveillance and incidence of AN among 1- vs 2-year surveillance intervals.
Data Analysis: Kaplan-Meier analysis.
Funding: Grants from the Dutch Cancer Society (KWF) and the Instituto de Salud Carlos III (PI16 /00766). Co-funded by the European Regional Development Fund (ERDF). CIBEREHD is funded by the Instituto de Salud Carlos III.
Results: Overall, 271 eligible patients were followed for a median of 3.6 years, with a mean age of 60 years old at the start of surveillance. Ninety-nine (36.5%) patients met SPS type I criteria, 99 (36.5%) met type III, and 73 (27%) met both type I & III. Sixty-seven (25%) patients had CRC prior to surveillance. At first surveillance, 131 (52%) received a 2-year follow-up recommendation and 140 (48%) received a 1-year recommendation. Among those with a 1-year follow-up, 50% remained on a 1-year program at second and third surveillance and 50% were transitioned to a 2-year follow-up. Most patients who were recommended a 2-year interval remained with this recommendation after the second (64%) and third (71%) surveillance.
The 5-year cumulative incidence of CRC was 1.3% (95% Confidence Interval [CI] 0-3.2%), which consisted of 2 cases both of whom had prior CRC/AN. The 5-year cumulative incidence of advanced neoplasia was 44% (95% CI 37%-52%). AN incidence was lower for SPS type III (26%) than type I (53%): hazard ratio = 0.38 (95% CI 0.22-0.63, P<0.001) adjusted for age, smoking status, and gender. There were no other significant risk factors for AN identified. There was no statistically significant difference in AN incidence between a 1- or 2-year surveillance interval. Surgery was only required for CRC or bowel adhesions in 3 patients during surveillance.
COMMENTARY
Why Is This Important?
SPS is one of the most common CRC-predisposition polyposis syndromes. The prevalence is approximately 1:111 among individuals > 50 years old with positive fecal immunochemical tests.2 The National Comprehensive Cancer Network (NCCN) guidelines recommend a 1-3 year interval range,3 while 2012 USMSTF guidelines recommend annual surveillance colonoscopy for SPS patients.4 While close colonoscopic surveillance is effective and safe,5 minimizing overuse of surveillance colonoscopy can alleviate system- and patient-level procedural burden. This study is one of the largest prospective cohorts of SPS patients undergoing colonoscopic surveillance and identifies SPS patients who can safely extend their surveillance interval to every 2 years.
In 2019 (after this study was completed), the WHO criteria for SPS were updated, most notably eliminating the former type II criteria in which any number of serrated polyps proximal to the sigmoid colon in an individual with a first-degree relative with SPS would themselves meet criteria for SPS (Figure 1). Given how prevalent serrated lesions are in the general population, this criteria had poor specificity for true SPS.1 Type I criteria was updated to include serrated lesions in the sigmoid colon due to evidence showing that up to 50% of CRCs in SPS occur in the rectosigmoid colon, but with a minimum size of 5 mm. Type III criteria were renamed as the new type II criteria and now requires at least 5 SSLs proximal to the rectum.
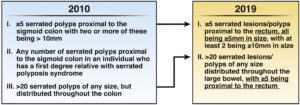
Figure 1. Changes in the World Health Organization’s 2010 and 2019 criteria for serrated polyposis syndrome diagnosis. Changes in 2010 I and II criterion are marked with underlining in 2019 criterion. Note that 2010 criterion II was not included with the 2019 criterion. Reused with permission from Gastroenterology.1
Key Study Findings
Caution
This surveillance study was conducted after all patients underwent clearing of all polyps ≥5 mm and <5 mm with optical appearance of an adenoma or serrated lesion with successful cecal intubation, adequate bowel preparation, and repeat colonoscopies within 6 months as necessary to complete polyp clearing. Virtual chromoendoscopy or distal attachment devices were also not standard practice in this study.
My Practice
After identifying a patient with SPS, I schedule the initial clearing colonoscopy within 3 months and then repeat every 3-6 months until there are <5 small/large polyps and no AN.6 In order to minimize missed polyps, I usually perform retroflexion in the right colon and use virtual chromoendoscopy (if excellent bowel prep) and a distal attachment device and cleanse residual stool to achieve excellent bowel cleansing. Thereafter, I transition to annual colonoscopies for 2-3 years before relaxing to a biannual interval, while taking into account colonoscopy quality and patient preference. I also recommend early screening of first-degree family members at age 40 (or earlier if early-onset CRC or AN) with repeat every 5 years if no polyps are found3 at multiple points of patient contact (clinic, colonoscopy report, pathology result letter).
Although SPS often has a familial component, SPS is typically not associated with a pathogenic genetic variant (rare cases can be caused by variants in RNF43). I do offer genetic testing based on patient preference or if there are concomitant adenomatous polyps, which raises the likelihood of mixed polyposis syndromes such as MUTYH-associated polyposis.3
It is important to remember that SSLs are sporadic in most patients in the absence of SPS, and SSLs with any dysplasia are thought to be comparable to adenomas with high-grade dysplasia in terms of CRC risk.7 However, as discussed in a previous EBGI summary, these polyps tend to be flat, subtle in appearance, and easily missed particularly in the proximal colon.8 There is recent data showing an inverse association between sessile serrated detection rates (SSLDR) and post-colonoscopy CRC risk,9 raising the question whether SSLDR should be considered a colonoscopy quality metric.10
For Future Research
In order to prevent CRC due to SPS, we must improve recognition of SPS patients, especially since the diagnosis is based upon lifetime total number of serrated polyps removed. In addition to improving provider knowledge, a flaw of our current healthcare system is the lack of a centralized longitudinal endoscopic record and cumulative polyp counter. Many patients obtain colonoscopies over time at a variety of clinical locations, and diagnosis of polyposis is often made either in the patient with a peculiar number of polyps during a single colonoscopy, or by an astute clinician paying particular attention to total number of serrated polyps removed during past colonoscopies.
Conflict of Interest
Dr. Yen reports no conflicts of interest.
REFERENCES
- Dekker E, Bleijenberg A, Balaguer F. Update on the World Health Organization Criteria for Diagnosis of Serrated Polyposis Syndrome. Gastroenterology 2020;158:1520-1523.
- JEG IJ, Bevan R, Senore C, et al. Detection rate of serrated polyps and serrated polyposis syndrome in colorectal cancer screening cohorts: a European overview. Gut 2017;66:1225-1232.
- Weiss JM, Gupta S, Burke CA, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 1.2021. J Natl Compr Canc Netw 2021;19(10):1122-1132.
- Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-857.
- Bleijenberg AGC, JEG IJ, Hazewinkel Y, et al. The long-term outcomes and natural disease course of serrated polyposis syndrome: over 10 years of prospective follow-up in a specialized center. Gastrointest Endosc 2020;92:1098-1107.e1.
- Mankaney G, Rouphael C, Burke CA. Serrated polyposis syndrome. Clin Gastroenterol Hepatol 2020;18:777-779.
- Sheridan TB, Fenton H, Lewin MR, et al. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas: an immunohistochemical study of serrated lesions “caught in the act.” Am J Clin Pathol 2006;126:564-71.
- Patel SG. Colonoscopy reduced CRC incidence and CRC-related mortality…if you get it! Evidence Based GI 2022; 2(8): https://gi.org/journals-publications/ebgi/patel_october2022/.
- Anderson JC, Rex DK, Mackenzie TA, et al. Higher Serrated Polyp Detection Rates Are Associated With Lower Risk of Postcolonoscopy Colorectal Cancer: Data From the New Hampshire Colonoscopy Registry. Am J Gastroenterol 2023;118:1927-1930.
- Ladabaum U. The Time Has Come to Adopt the Sessile Serrated Lesion Detection Rate as a Quality Metric. Am J Gastroenterol 2023; 118(11): 1954-56.

